Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
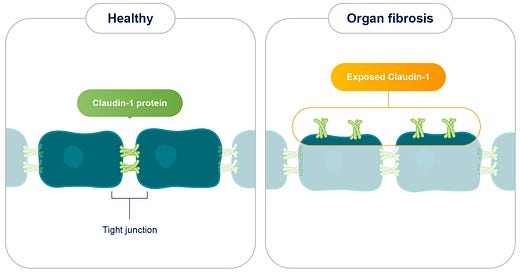
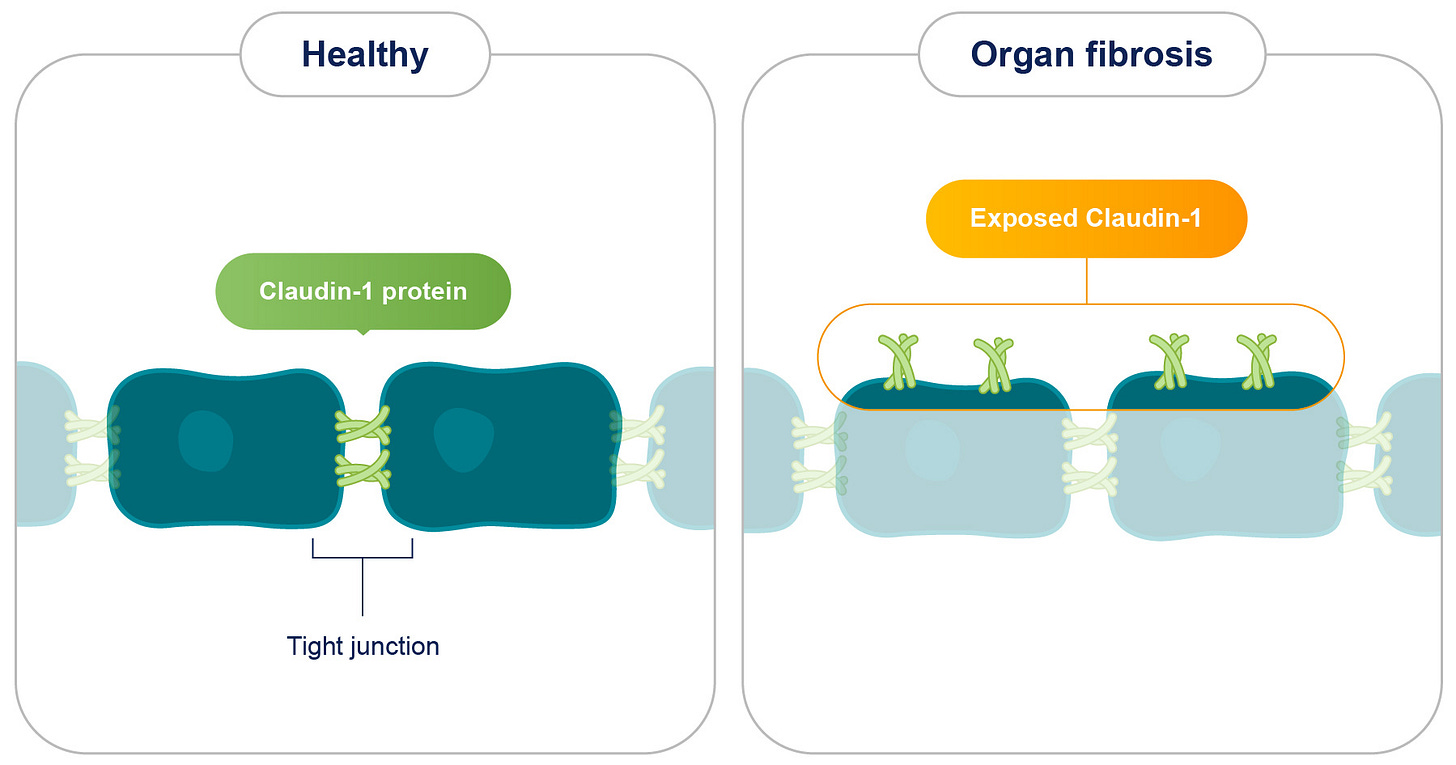
Fibrosis, a debilitating condition characterized by the excessive accumulation of extracellular matrix (ECM) components, poses a significant global health challenge, contributing to a substantial proportion of deaths, particularly in developed nations. It affects various organs, including the kidneys, liver, and lungs, leading to organ dysfunction and potentially culminating in life-threatening complications like cancer. Despite its widespread impact, current therapeutic options for fibrosis remain limited, emphasizing the urgent need for novel treatment strategies. Recent research has unveiled a promising therapeutic target for fibrotic diseases: Claudin-1 (CLDN1), a protein typically residing within tight junctions and crucial for regulating cell-to-cell adhesion.
In healthy tissues, CLDN1 is sequestered within tight junctions, effectively concealed from the extracellular environment. However, in fibrotic tissues, CLDN1 is overexpressed and aberrantly exposed outside of tight junctions, a phenomenon termed non-junctional CLDN1 (njCLDN1). This exposed njCLDN1 acts as a potent trigger for pro-fibrotic signaling pathways, driving the excessive deposition of collagen and other ECM components, ultimately leading to organ failure.
Numerous studies have established a compelling correlation between elevated CLDN1 expression and the severity of fibrosis across various organs. For instance, in individuals with idiopathic pulmonary fibrosis (IPF), CLDN1 expression escalates with disease progression, signifying its potential role in disease pathogenesis. Similarly, in liver tissues from patients with chronic liver diseases like HBV, HCV, or NASH, CLDN1 expression is markedly elevated, with njCLDN1 levels being particularly pronounced in advanced liver fibrosis. This observation underscores the potential of njCLDN1 as a valuable biomarker for fibrosis progression and a target for therapeutic intervention.
Advanced techniques like scRNA-seq analyses have provided a more nuanced understanding of CLDN1 expression patterns within the intricate cellular landscape of fibrotic tissues. In the context of liver fibrosis, CLDN1 exhibits high expression in a variety of cell types implicated in the fibrotic process, including hepatocytes, cholangiocytes, and activated hepatic stellate cells (aHSCs), the key players in hepatic collagen production. Notably, njCLDN1 is preferentially enriched in a distinct subpopulation of diseased hepatocytes situated at the junction between epithelial and stromal tissues, hinting at its involvement in the cellular transformations that drive fibrosis.
While the precise mechanisms by which njCLDN1 orchestrates the fibrotic cascade are still under investigation, several pivotal pathways have been brought to light. One prominent mechanism involves the activation of pro-fibrotic signaling pathways. njCLDN1 interacts with various cell membrane proteins, including EGFR and ITGA5, triggering downstream signaling cascades like the SRC and MAPK pathways, well-established drivers of fibrosis. Moreover, njCLDN1 appears to exert a modulatory influence on the TGF-β signaling pathway, a central regulator of fibrosis. Although it doesn't significantly impact the canonical SMAD2/3 pathway of TGF-β signaling, njCLDN1 effectively inhibits non-canonical pathways such as AKT, p38, and ERK signaling, known to be involved in fibrosis.
Adding another layer of complexity, njCLDN1 is upregulated by the TNF-α signaling pathway, a critical mediator of inflammation and fibrosis. In vitro experiments have convincingly demonstrated that exposing aHSCs and hepatocytes to TNF-α results in increased njCLDN1 expression, a process mediated by the NF-κB pathway, a downstream effector of TNF-α signaling.
Beyond its role in signaling pathways, njCLDN1 also plays a crucial role in modulating cell-matrix interactions and cell plasticity. Its interaction with ECM components like laminin suggests its involvement in the excessive deposition of ECM components, a hallmark of fibrosis. Furthermore, the association between njCLDN1 expression and alterations in cell plasticity, particularly in hepatocytes and liver progenitor cells, implies its contribution to the de-differentiation of hepatocytes and the proliferation of progenitor cells, processes intimately linked to fibrosis progression.
The therapeutic potential of targeting njCLDN1 has been strongly supported by a series of compelling preclinical studies. One notable study demonstrated that in vivo knockdown of CLDN1 using siRNA technology effectively mitigated liver fibrosis in a mouse model of NASH. Mice treated with CLDN1-targeting siRNAs exhibited significantly reduced collagen accumulation and a lower number of liver nodules compared to their control counterparts.
Further bolstering the therapeutic promise of targeting njCLDN1, monoclonal antibodies specifically designed to neutralize njCLDN1 have shown remarkable anti-fibrotic effects in various preclinical models. For example, in a humanized mouse model mimicking NASH-associated liver fibrosis, administration of a CLDN1 mAb effectively reduced liver fibrosis and suppressed the expression of fibrosis markers. Similar anti-fibrotic benefits were observed in a mouse model of biliary fibrosis treated with the same CLDN1 mAb.
Encouragingly, the therapeutic efficacy of CLDN1 mAbs extends beyond the liver, underscoring its broad applicability. Studies employing a mouse model of kidney fibrosis induced by unilateral ureteral obstruction and a mouse model of lung fibrosis induced by bleomycin have unequivocally shown that CLDN1 mAb treatment significantly attenuates fibrosis in these organs, highlighting its potential as a pan-fibrotic therapy.
These preclinical findings converge to paint a compelling picture: targeting njCLDN1 with precisely engineered mAbs represents a potentially safe and effective strategy for combating fibrosis across diverse organs. Importantly, toxicity studies conducted in non-human primates have revealed that CLDN1 mAbs are well-tolerated and do not disrupt the integrity of tight junctions, reinforcing their safety profile.
In conclusion, CLDN1 has emerged as a highly promising target for the development of innovative anti-fibrotic therapies. Its overexpression and aberrant exposure outside of tight junctions in fibrotic tissues, coupled with its pivotal roles in pro-fibrotic signaling pathways and cellular processes, render it an attractive candidate for therapeutic intervention. The robust anti-fibrotic effects observed in various animal models treated with CLDN1-targeting siRNAs and mAbs strongly support the translational potential of this approach, paving the way for clinical development.
Looking ahead, future research endeavors should prioritize several key areas:
Delineating the precise molecular mechanisms by which njCLDN1 contributes to fibrosis in different organs. A deeper understanding of these mechanisms will enable the development of more targeted and effective therapies.
Optimizing the delivery and efficacy of CLDN1-targeting therapies. This may involve exploring novel drug delivery systems or developing next-generation CLDN1 mAbs with enhanced potency and target specificity.
Initiating clinical trials to rigorously evaluate the safety and effectiveness of CLDN1-targeting therapies in patients with fibrotic diseases. This crucial step will determine the clinical translatability of this promising therapeutic approach.
The successful development of safe and effective therapies targeting njCLDN1 holds immense promise for addressing the substantial unmet medical need in the management of fibrotic diseases. By disrupting the fibrotic cascade and restoring tissue homeostasis, CLDN1-targeted therapies have the potential to revolutionize the treatment landscape for fibrosis, offering hope for improved patient outcomes and a brighter future for those battling this debilitating condition.