Synthetic integrin antibodies discovered by yeast display reveal αV subunit pairing preferences with β subunits
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
The research paper uses yeast display to generate a panel of highly specific and functional antibodies against key integrin heterodimers. Beyond the technical advancements in antibody discovery, the paper also provides new insights into the subunit pairing preferences of the integrin αV subunit, a topic that is crucial to understanding the roles and functionalities of this receptor family.
Integrins are a family of cell surface receptors that mediate crucial cellular processes, including cell adhesion, migration, and intracellular signaling. They are heterodimeric glycoproteins, consisting of α and β subunits, with diverse combinations allowing for specific interactions with various extracellular ligands. Eight members of this large family of 24 integrins recognize the tripeptide Arg-Gly-Asp (RGD) motif, which is present in a wide array of extracellular matrix proteins and cell surface proteins. These so-called RGD-binding integrins, including αVβ3, αVβ5, αVβ6, αVβ8, and α5β1, are implicated in numerous physiological and pathological conditions, making them attractive targets for therapeutic interventions. Despite the similar RGD-binding motif, each integrin demonstrates distinct ligand specificities and cellular functions, highlighting the need for selective targeting.
The authors begin by addressing a persistent challenge in the field of integrin research: the lack of highly specific antibodies that can directly block the interaction of integrins with small RGD-containing ligands. Many antibodies to date have been shown to interact outside the ligand-binding pocket, impacting only the binding of large macromolecules. In contrast, this study successfully engineered antibodies that compete with small RGD peptides for integrin binding, demonstrating their specificity and potential for therapeutic development. They achieve this through the use of a synthetic yeast-displayed Fab antibody library, a technology that offers several advantages over traditional immunization-based methods. Namely, the library is capable of generating antibodies against both human and mouse antigens, and is less subject to the self-tolerance mechanisms of immune systems, expanding the library's sequence space and increasing the likelihood of discovering high-affinity antibodies.
The research team employed a combination of magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) to select yeast clones displaying Fabs that specifically bound to the target integrin heterodimers. They targeted five RGD-binding integrins: αVβ3, αVβ5, αVβ6, αVβ8, and α5β1. Their careful screening strategy included positive selection with purified integrin ectodomains and negative selection with untargeted integrins and a poly-specificity reagent to avoid cross-reactive antibodies. This meticulous approach resulted in the identification of 11 antibodies, all exhibiting high specificity and affinity for their respective target integrins. Of these 11, six had a ligand-mimetic R(G/T/L)D motif in their complementarity-determining region (CDR3), a structural feature that allows for more efficient binding competition with RGD-containing ligands.
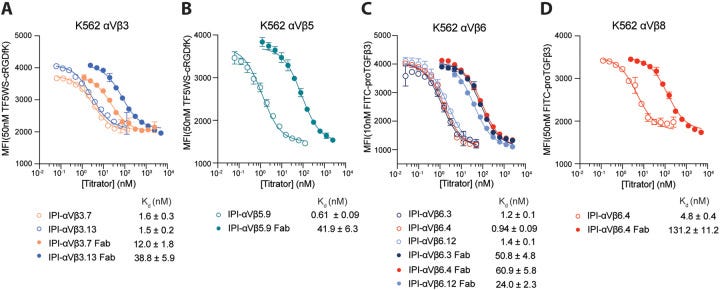
The next stage of the work is focused on the characterization of these antibodies. Initially, the team performed immunofluorescence staining on cells expressing intact human and mouse integrins, assessing antibody specificity and cross-reactivity. The data showed that the vast majority of these antibodies were highly selective for the integrins that were used in the selection process, while also displaying significant binding to their mouse orthologs. The binding affinity was further characterized through surface plasmon resonance (SPR). Here, purified integrin ectodomains were exposed to immobilized antibodies to determine association and dissociation rates, and dissociation constants (Kd) ranging from sub-nanomolar to double-digit nanomolar affinities were determined. These results highlighted the high-affinity binding of all 11 antibodies toward their targeted integrin, as well as their remarkable selectivity.
To further examine the function of these antibodies, the team conducted a fluorescence polarization (FP) competition assay with fluorescent RGD-mimetic peptide ligands. This analysis confirmed that all six RGD-motif-containing antibodies indeed compete with peptide ligands for integrin binding. It also revealed significant cross-reactivity of some of the antibodies towards untargeted integrins, albeit with several magnitudes lower affinity compared to the target integrins. This particular result confirms that many of these antibodies are capable of directly engaging the RGD-binding pocket of the integrins and effectively block the binding of small-molecule antagonists. Importantly, these results support the hypothesis that incorporation of a ligand-mimetic motif in the antibody’s CDR3 loop can facilitate this mechanism.
Furthermore, the authors examined the apparent affinity of these antibodies on cell surfaces, an assessment that reflects avidity and is most pertinent to biological systems. Using flow cytometry without washing, the group measured antibody binding to cell surface integrins and determined dissociation constants for each of the RGD-mimetic antibodies. The results were notable for a strong avidity effect; antibodies in the IgG format bound with a significantly higher effective affinity than Fab fragments of the same antibody, demonstrating that IgG’s two antigen-binding sites lead to enhanced binding to cell surfaces. They also demonstrated the efficacy of the blocking activity of these antibodies, especially with the use of a cell adhesion assay. In a similar vein, this analysis showed that the RGD-motif-containing antibodies were effective at blocking cell attachment to fibronectin.
One of the most important discoveries described by the authors is related to the binding preferences of the αV subunit to the five different β subunits with which it can associate. This is highly relevant to the biology of αV integrins given that it determines the specific roles of each heterodimer in cellular signaling, development, and disease. By transiently expressing αV with different ratios of each of the five β-subunits, the authors used their panel of antibodies to study the preferences of αV for the different β-subunits. By titrating the concentration of a given β subunit relative to the αV subunit, the authors could precisely map the preference of each of these heterodimers. Through these experiments, the authors revealed a consistent preference hierarchy for αVβ interactions: β6=β8>β3>β1=β5. This finding provides important new insights into how these heterodimers are formed in cells and has significant implications for understanding their distinct roles in biology. The authors also showed that this hierarchy was observed not just in transfected cells, but also in endogenous integrins expressed on tumor cell lines.
Finally, the authors compare these antibodies to previously reported antibodies. They note that most antibodies that have been reported to date do not directly bind to the ligand-binding pocket, and are therefore incapable of competing with small molecules that block integrin activity. Moreover, the only antibodies known to block small molecule binding to integrin are those that target αIIbβ3. The antibodies described in this manuscript represent novel approaches, showing that antibodies that directly compete with small molecule ligands are obtainable via engineered Fab libraries. These results establish the potential of these antibodies for integrin targeting.
In summary, this paper provides significant advances in several important areas of integrin research. The generation of a new panel of antibodies, targeted against specific RGD-binding integrins and capable of blocking the activity of small molecule RGD-mimetic antagonists, represents a key advancement that will facilitate further research in this space. Furthermore, by mapping the subunit pairing preferences of the integrin αV subunit, this work gives insight into the biological activity of these critical receptors. The methods described in this paper are rigorous and thorough, using a combination of in vitro, cell-based, and in vivo assays to characterize their newly developed antibodies. The conclusions are well supported by the data and contribute significantly to our understanding of integrin function.
Moving forward, this study has created several opportunities for the use of these antibodies in biological studies and for potential therapeutic development. The authors acknowledge the therapeutic potential of integrin inhibition, and the data presented here suggest that their antibodies are well suited for this application. Their successful development of antibodies that block the binding of small molecules and proteins within the integrin ligand-binding pocket opens new avenues for developing more effective and specific therapeutics. The authors, recognizing the value of these reagents, are now distributing these antibodies in collaboration with the IPI and Addgene, further bolstering their impact on the research community.
This work on synthetic integrin antibodies presents a valuable resource for the research community, providing tools that directly compete with small-molecule antagonists and elucidate subunit pairing preferences within the integrin family. This research pushes forward our comprehension of the intricacies of integrin biology and unlocks new possibilities for therapeutic interventions.