Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
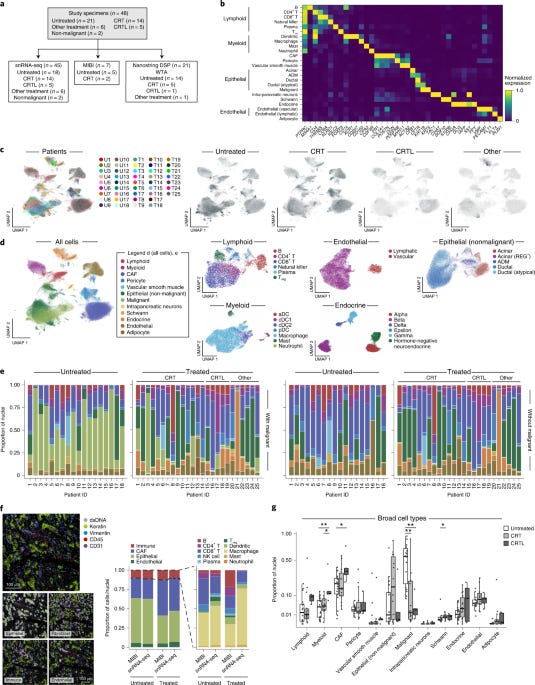
From the Aviv Regev lab, the paper uses single-nucleus RNA sequencing and spatial transcriptomics to characterize the composition and molecular profiles of 43 primary pancreatic ductal adenocarcinoma (PDAC) tumors, including 18 untreated and 25 treated with neoadjuvant chemotherapy and/or radiotherapy.
Identifying 33 cell types encompassing malignant, epithelial, immune, and other stromal cells. Malignant cells showed genomic alterations consistent with PDAC. With evidence supporting an acinar-to-ductal metaplasia (ADM) transitional state and atypical ductal cells as intermediates in the progression from normal epithelium to PDAC.
Discovering 14 malignant and 4 cancer-associated fibroblast (CAF) expression programs beyond previously defined subtypes, including a neural-like progenitor program enriched after treatment. The neural-like progenitor program was associated with poor prognosis and contains genes linked to perineural invasion, a common occurrence in PDAC. With this, three spatially-defined multicellular communities were found, one of which ("treatment-enriched") features the neural-like progenitor program, neurotropic CAFs, and CD8+ T cells. Along with multiple ligand-receptor interactions correlated across cell compartments specifically in treated tumors represent potential therapeutic targets. This refined taxonomy of PDAC composition and states provides a valuable framework for understanding tumor heterogeneity, evolution, microenvironment interactions, and clinical outcomes in pancreatic cancer.