Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
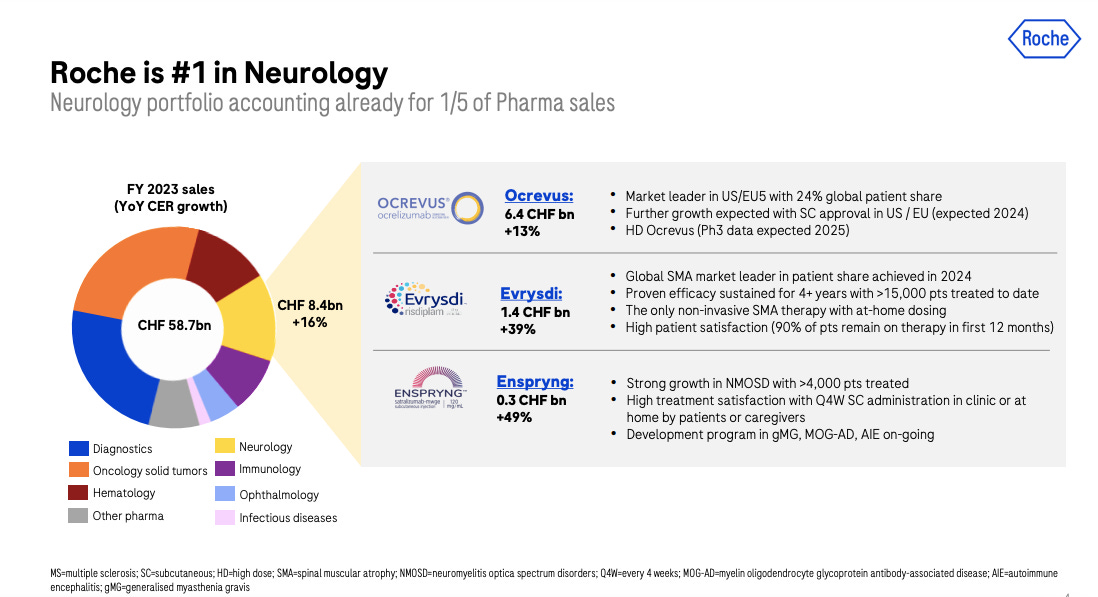
Roche’s neurology strategy is betting on the next Alzheimer's treatment to maintain its current lead. And to add to their broad neurology pipeline spanning neuromuscular, neuroinflammatory, and neurodegenerative diseases, using engineered therapeutic modalities like gene therapies and brain shuttle antibodies:
1. Ocrevus - the multiple sclerosis drug is expecting approvals for subcutaneous dosing (2024) and a higher dose formulation (2025 data expected).
2. Fenebrutinib - selective brain-penetrant BTK inhibitor being studied in relapsing and progressive MS, with Phase 3 data expected in 2025. Phase 2 data showed significant reductions in brain lesions.
3. Enspryng - IL-6 inhibitor approved for NMOSD, being studied in generalized myasthenia gravis (Phase 3 data H1 2024), autoimmune encephalitis and MOG antibody disease.
4. Evrysdi + GYM329 - Evrysdi is Roche's approved SMA therapy. GYM329 is an anti-myostatin antibody being studied to improve muscle strength when combined with Evrysdi in SMA (interim Phase 2 data in 2024).
5. Elevidys - micro-dystrophin gene therapy approved in the US for ambulatory DMD patients aged 4-5 years. Phase 3 primary results at 1 year showed potential for disease trajectory modification.
6. Trontinemab - a brain shuttle antibody against amyloid-beta that demonstrated rapid, dose-dependent amyloid plaque reduction in early Alzheimer's disease studies. Phase 3 enabling data expected in 2024.
7. Diagnostics - focused on blood-based Alzheimer's screening and respiratory panels.
Alzheimer's disease (AD) affects millions of people worldwide. Despite facing significant setbacks with previous drug candidates, Roche remains committed to its neurology research and development program, particularly in AD. The company's latest hope lies in an experimental drug called trontinemab, which belongs to a new class of therapies designed to penetrate the brain more effectively and clear the clumps of amyloid protein believed to be a major contributing factor to Alzheimer's disease. The drug use a novel delivery technology (i.e. Brainshuttle) that enhances its ability to cross the blood-brain barrier, a critical challenge in treating neurological disorders.
Recently, Roche shared promising early data from a small clinical trial involving trontinemab. According to the company, the highest dose of the drug demonstrated "best-in-class" potential, with the majority of patients in that dosage group achieving undetectable levels of amyloid in their brains after just 12 weeks of treatment.
This rapid clearance of amyloid plaques is a remarkable achievement, especially when compared to the performance of other Alzheimer's drugs currently on the market or in late-stage development. Biogen and Eisai's Leqembi, for instance, has shown the ability to slow cognitive decline in Alzheimer's patients, but its effects on amyloid clearance are more modest, and the treatment has been associated with a higher risk of a side effect known as ARIA (Amyloid-Related Imaging Abnormalities).
Roche is positioning trontinemab as a potential game-changer in the Alzheimer's treatment landscape, emphasizing its potential to offer a more efficient and potentially safer alternative to existing therapies. If the drug's impressive early results can be replicated in larger clinical trials, it could become a formidable competitor in a market where there is a significant unmet need for effective and well-tolerated treatments. This rapid clearance of amyloid plaques could open up new possibilities for treatment regimens, such as reducing or stopping therapy once a patient's amyloid levels have been brought down to undetectable levels. But it is too early to say whether such a strategy would lead to sustained clinical benefits. However the potential for less frequent dosing or shorter treatment durations is an intriguing possibility that could further differentiate trontinemab from its competitors.
While the early data on trontinemab is undoubtedly encouraging, Roche remains cautious and recognizes that there is still a long road ahead before the drug can potentially reach the market. The company is currently expanding the early-stage trial to enroll a couple hundred more patients, with the aim of gathering more comprehensive data on the drug's safety, efficacy, and optimal dosing regimen.
Roche's pursuit of trontinemab and its continued investment in Alzheimer's research stem from a renewed faith in the amyloid hypothesis – the theory that the buildup of amyloid plaques in the brain is a primary driver of the neurodegenerative process in Alzheimer's disease. This hypothesis was called into question following a series of high-profile clinical failures involving drugs designed to target amyloid, leading many researchers to question whether this approach was indeed viable. However, the recent success of Biogen and Eisai's Leqembi, as well as Eli Lilly's donanemab, has reignited interest in the amyloid hypothesis and provided fresh impetus for companies like Roche to continue exploring this therapeutic avenue.
Roche's commitment to Alzheimer's research is driven not only by the immense scientific challenge posed by the disease but also by the growing societal and economic burden it represents. As the global population ages, the prevalence of Alzheimer's and other forms of dementia is expected to rise dramatically, placing tremendous strain on healthcare systems and caregivers. By developing an effective and potentially superior treatment for Alzheimer's, Roche stands to make a significant impact on patient outcomes and quality of life, while also capturing a substantial share of a market that is projected to grow rapidly in the coming years.
Beyond trontinemab, Roche's neurology pipeline includes several other promising candidates targeting various neurological conditions. The company is also exploring alternative therapeutic approaches, such as gene therapy and regenerative medicine, in an effort to address the complex and multifaceted nature of neurodegenerative disorders. In parallel with its drug development efforts, Roche has been actively pursuing partnerships and collaborations with academic institutions, biotechnology companies, and other stakeholders in the neuroscience field. These collaborative endeavors aim to accelerate the translation of scientific discoveries into novel therapeutic solutions and to foster a greater understanding of the underlying mechanisms driving neurological diseases.