Protein stability promotes evolvability
Analysis
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
The classic paper investigates how protein stability affects the capacity of proteins to evolve new or improved functions. The authors use computational simulations and lab experiments on P450 enzymes to demonstrate that extra thermodynamic stability enhances a protein's evolvability by allowing it to tolerate a wider range of destabilizing but functionally beneficial mutations.
Starting by discussing how the biophysical properties enabling the remarkable evolvability of proteins are largely unknown, the work introduces the hypothesis that a protein's robustness to mutations, conferred by extra stability, contributes to its capacity to evolve.
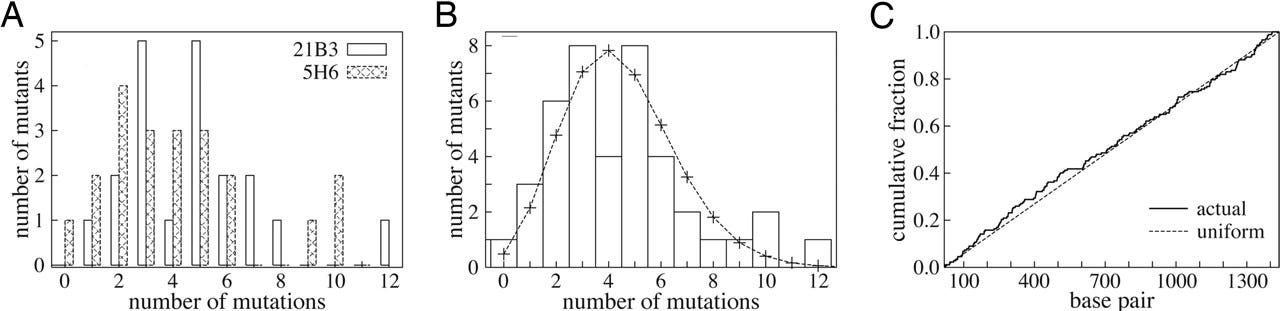
To test this idea, the authors first performed simulations using simplified lattice protein models. They evolved a model lattice protein to stably fold and bind to a ligand. They then created a stabilized variant and found that, compared to the original protein, it produced over twice as many improved mutants with affinity for new ligands. Analysis showed the extra stability allowed more mutants to fold correctly, and the improved mutants tended to be destabilized.
The authors then experimentally tested how stability affects evolvability of real P450 enzyme variants. They used error-prone PCR to create mutant libraries of a marginally stable P450 variant and a stabilized variant. When screening over 8,000 mutants from each library for new activities, the stabilized variant yielded over 3 times more improved mutants. Some mutants were greatly destabilized but only the stabilized parent could tolerate the destabilizing changes needed to confer novel activities.
Overall, the computational and experimental results establish that extra protein stability enhances evolvability by allowing proteins to accept a wider range of beneficial but destabilizing mutations while retaining fold and function. The findings reveal stability as a key determinant of a protein's capacity to evolve.
The paper combines computational modeling and real protein experiments to provide compelling evidence for the role of stability in promoting evolvability. The consistent results build confidence in the conclusions. The use of simplified lattice protein models provides an intuitive framework to understand the basic biophysical principles before validating on real proteins. Testing the hypothesis on an important protein family (P450 enzymes) shows the concept's relevance for engineering industrially useful proteins. The results have significant implications for both understanding natural protein evolution and designing better directed evolution strategies by stabilizing proteins first.
The lattice protein simulations, while illustrative, may be too simple to quantify stability effects on promoting functional innovations versus just robustness. The number of improved P450 mutants, while clearly higher for the stabilized variant, is still relatively small. Additional replicate experiments could strengthen conclusions. It remains unclear if stabilizing mutations found in nature similarly enhance evolvability or if there are other mechanisms to buffer destabilization.
Overall, the research is a foundational evidence that extra protein stability enhances the capacity of proteins to evolve new functions by better tolerating destabilizing mutations. By elucidating stability as a key determinant of protein evolvability, the work advances understanding of the forces shaping natural molecular evolution while also guiding protein engineering tactics.