Preclinical proof of principle for orally delivered Th17 antagonist miniproteins
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
This groundbreaking research paper presents a novel approach to developing oral therapeutics for autoimmune and inflammatory diseases, focusing on the computational design and preclinical testing of miniprotein inhibitors targeting the interleukin-23 receptor (IL-23R) and interleukin-17 (IL-17). The study aims to address the limitations of existing antibody therapies, which require injection and have various drawbacks, by creating small, stable protein inhibitors that can be administered orally.
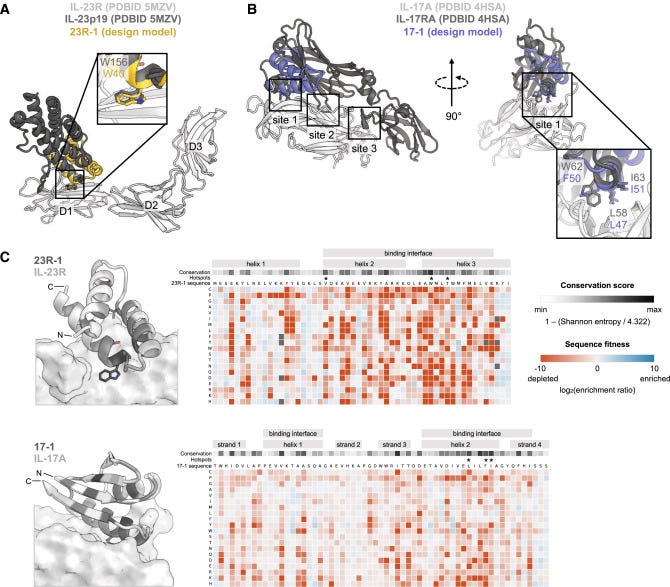
The researchers employed advanced computational protein design methods to create miniproteins, approximately 60 amino acids in length, capable of binding with high affinity to IL-23R and IL-17A. These initial designs incorporated both native "hotspot" residues from the natural ligands and computationally predicted binding residues. The designs were then subjected to experimental testing using yeast surface display and selected for their ability to bind to the target proteins.
Following the initial design phase, the most promising candidates underwent several rounds of in vitro evolution and additional computational design. This iterative process led to dramatic improvements in both binding affinity and stability of the miniproteins. For the IL-23R binders, the optimization resulted in a variant named 23R-91, which achieved sub-picomolar affinity with an unmeasurably slow dissociation rate. The IL-17A binders were enhanced by creating a dimeric construct called 17-53, capable of binding both sides of the IL-17A homodimer. This dimeric approach resulted in a remarkable 2,800-fold increase in potency compared to the monomeric binder.
To validate the computational designs, the researchers performed extensive structural characterization. The crystal structure of 23R-91 was solved and found to closely match the predicted model, with only 0.4-0.7 Å root mean square deviation in the backbone. This close alignment between the computational model and the actual structure provides strong evidence for the accuracy of the design process. Further validation came from saturation mutagenesis experiments, where every amino acid was systematically mutated to all other possible amino acids. The results of these experiments corroborated that the miniproteins were folded and binding as designed, lending additional credibility to the computational approach.
A critical consideration for oral therapeutics is their ability to withstand the harsh conditions of the gastrointestinal tract. The designed miniproteins demonstrated exceptional stability, resisting denaturation at high temperatures exceeding 95°C and maintaining their structure in the presence of chemical denaturants. Moreover, they showed remarkable resistance to proteases found in simulated gastric and intestinal fluids. To further enhance their stability, the researchers computationally designed and introduced intramolecular disulfide bonds. This additional feature contributed to the miniproteins' ability to survive the challenging environment of the digestive system, a crucial attribute for oral administration.
The efficacy of the miniproteins was thoroughly evaluated in various in vitro assays. In cell-based experiments, the IL-23R binders effectively blocked IL-23-mediated signaling with high potency. The dimeric IL-17A binder, 17-53, demonstrated even more impressive results, showing 200-fold greater potency than secukinumab, a clinical antibody used in the treatment of psoriasis. The researchers also tested the miniproteins in more physiologically relevant systems to better approximate their potential effectiveness in the human body. These tests included inhibition of cytokine signaling in primary human T cells and in organoids derived from human skin cells. The positive results in these more complex systems provide stronger evidence for the potential therapeutic value of the miniproteins.
To assess the feasibility of oral delivery, the researchers conducted pharmacokinetic studies in rats. After oral administration, the IL-23R miniproteins reached nanomolar concentrations in intestinal tissues, demonstrating their ability to survive the digestive process and penetrate the gut lining. Importantly, low levels of the miniproteins were also detected in serum, indicating some degree of systemic absorption. This finding is crucial, as it suggests that orally administered miniproteins could potentially reach targets beyond the gastrointestinal tract. The researchers observed similar uptake patterns in both healthy rats and those with experimentally disrupted intestinal barriers. This consistency across different gut conditions suggests that the miniproteins could be effective in both active disease states and as maintenance therapy.
The most compelling evidence for the potential of these miniproteins as oral therapeutics came from a study using a humanized mouse model of colitis. In this sophisticated model, immunodeficient mice are engrafted with human immune cells from inflammatory bowel disease patients and then challenged to induce colitis-like symptoms. The researchers compared the efficacy of orally administered 23R-91 to intraperitoneally injected guselkumab, a clinical anti-IL-23 antibody. Remarkably, the orally delivered 23R-91, at doses of 8 or 80 mg/kg daily, was as effective as the injected antibody in reducing disease scores. This finding is particularly significant as it demonstrates that the oral miniprotein could reach therapeutically relevant concentrations beyond the gut barrier to impact systemic inflammation.
The researchers highlight several potential advantages of their miniprotein approach over existing therapies. The small size of the miniproteins (7-8 kDa) compared to antibodies (150 kDa) may allow for better tissue penetration and oral bioavailability. Their high stability enables oral administration without the need for complex formulations or delivery technologies, which could simplify manufacturing and reduce costs. Unlike chemically synthesized peptides, which often require non-natural amino acids or modifications for stability, these miniproteins are genetically encodable. This characteristic allows for simpler and potentially cheaper manufacturing using standard recombinant protein production methods.
Another potential advantage of the miniproteins is their predicted low immunogenicity. Based on their high stability, resistance to proteolysis, and in silico analysis of potential T cell epitopes, the researchers anticipate that these miniproteins are less likely to provoke an immune response than other biological therapies. This could translate to a better safety profile and sustained efficacy over long-term treatment.
Despite the promising results, the paper also discusses some limitations and areas for further investigation. While 23R-91 showed efficacy with once-daily oral dosing in the colitis model, pharmacokinetic data indicated rapid clearance from the blood after absorption. The researchers propose that the extremely high affinity and slow dissociation rate of the miniprotein from its target may allow for sustained inhibition even as free drug levels decline. However, this hypothesis requires further study to confirm the duration of target engagement and to optimize dosing regimens.
The doses used in both pharmacokinetic and efficacy studies were relatively high, reaching up to 140 mg/kg in rats. The authors acknowledge this limitation but suggest that lower doses may be achievable in clinical applications by using solid oral dosage forms such as tablets or capsules. These formulations could potentially increase local drug concentration in the intestine compared to the liquid formulations used in the preclinical studies, thereby improving bioavailability and reducing the required dose.
The researchers also touch on the potential broader applications of their approach. While this study focused on IL-23R and IL-17A as targets for inflammatory bowel disease and psoriasis, the computational design method could potentially be applied to a wide range of protein targets. This versatility suggests that the miniprotein approach could be adapted to address various diseases where protein-protein interactions play a key role.
The paper concludes by emphasizing the potential of computationally designed miniproteins as a new modality for oral biologic therapies. The ability to create small, stable proteins with antibody-like potency that can be delivered orally could address many of the limitations of current antibody therapies for autoimmune and inflammatory diseases. This approach opens up new possibilities for more convenient and potentially less expensive treatment options for patients with chronic inflammatory conditions.
However, the authors are careful to note that further development and clinical testing will be necessary to fully realize the potential of this approach. Questions remain about the long-term safety and efficacy of these miniproteins in humans, as well as the optimal formulation and dosing strategies. Additionally, while the computational design methods have proven successful in this case, their broader applicability and reliability for targeting other proteins will need to be demonstrated.
In summary, this research represents a significant advance in the field of protein therapeutics. By combining cutting-edge computational design with rigorous experimental validation and in vivo testing, the researchers have demonstrated a promising new approach to creating oral biologics. If further developed and successfully translated to the clinic, this technology could revolutionize the treatment of autoimmune and inflammatory diseases, offering patients more convenient, effective, and potentially more affordable treatment options. Moreover, the principles and methods developed in this study could pave the way for a new generation of orally available protein therapeutics targeting a wide range of diseases.
As the field of computational protein design continues to advance, we can expect to see further refinements and applications of this approach. Future research may focus on expanding the range of targetable proteins, improving the predictability of in vivo behavior, and optimizing manufacturing processes for large-scale production. Additionally, as our understanding of the factors influencing oral bioavailability of proteins grows, we may see even more efficient designs that can achieve therapeutic effects at lower doses.
The success of this approach also highlights the power of interdisciplinary research in addressing complex medical challenges. By bringing together expertise in computational biology, protein engineering, pharmacology, and immunology, the researchers were able to tackle a problem that has long eluded traditional drug discovery approaches. This collaborative model of research is likely to become increasingly important as we seek to develop more sophisticated and targeted therapies for a wide range of diseases.
In conclusion, this paper presents a significant step forward in the development of oral biologic therapies. While there is still work to be done before these miniproteins can be used in clinical practice, the results presented here offer a compelling proof of concept and a roadmap for future development. As this technology matures, it has the potential to significantly improve the lives of patients with chronic inflammatory conditions, offering new hope for more effective and convenient treatments.