Immobility-associated thromboprotection is conserved across mammalian species from bear to human
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
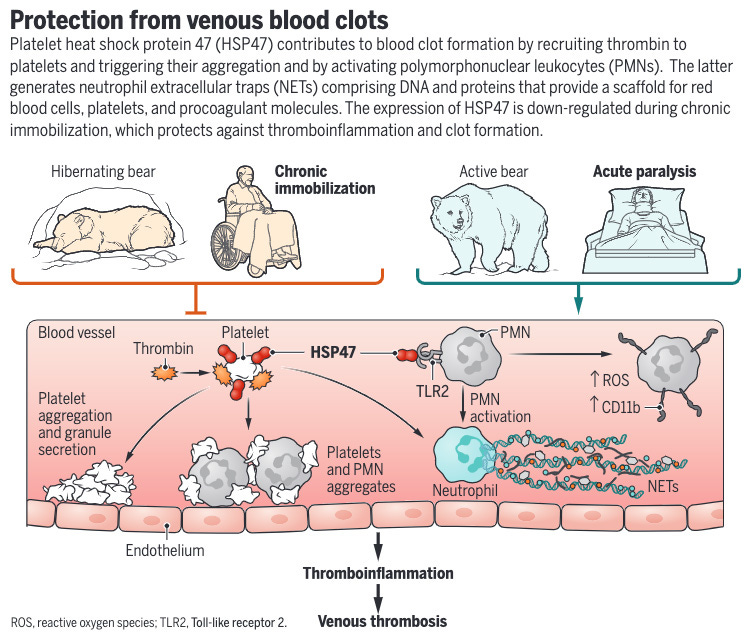
Hibernating brown bears and chronically paralyzed spinal cord injury (SCI) patients are protected from immobility-associated venous thromboembolism (VTE). The mechanisms behind this protection were unknown. The researchers performed a cross-species analysis using brown bears, mice models, humans, and pigs. They found an antithrombotic molecular signature in platelets during hibernation and chronic paralysis.
The most substantially reduced platelet protein in hibernating bears was heat shock protein 47 (HSP47). Knockout or inhibition of HSP47 in mice reduced thrombosis and inflammation. SCI patients had reduced platelet HSP47 compared to controls. This was associated with reduced thrombin binding, neutrophil activation, and neutrophil extracellular trap (NET) formation.
Healthy volunteers undergoing bed rest also showed platelet HSP47 downregulation. HSP47 reduction appears to be a conserved anti-thrombotic mechanism across mammalian species during immobilization. Targeting platelet HSP47 may lead to new therapies to prevent immobility-associated VTE. The findings also provide insight into why chronically paralyzed patients do not have excessive VTE risk despite immobilization.
In summary, this cross-species study identified a conserved platelet signature that protects against thrombosis during immobilization, with HSP47 downregulation as a key mechanism. This advances our understanding of thrombosis regulation and may enable new therapeutic approaches.