Discovery of a new class of integrin antibodies for fibrosis
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
Fibrosis, the excessive accumulation of extracellular matrix proteins like collagen in tissues, is a hallmark of many chronic diseases affecting organs such as the lungs, liver, and kidneys. Idiopathic pulmonary fibrosis (IPF) is a particularly devastating form of lung fibrosis, with a median survival of only 2.5-3.5 years after diagnosis. Current treatments like pirfenidone and nintedanib slow disease progression but do not halt or reverse fibrosis. There is an urgent need for more effective therapies.
A new study by explores a promising therapeutic approach: inhibition of αv integrins. Integrins are cell surface receptors that mediate cell-extracellular matrix interactions and play key roles in fibrosis development. This work provides further validation for targeting αv integrins and introduces novel antibodies with unique properties that could advance the field.
The small molecule pan-αv integrin inhibitor MK-0429 reduced fibrosis progression in a mouse model of lung injury. A set of new monoclonal antibodies against αv integrins were discovered with cross-reactivity to both human and mouse proteins. One antibody, Ab-31, potently inhibited multiple αv integrin functions including ligand binding, cell adhesion, and TGFβ activation. Ab-31 showed high potency in suppressing TGFβ-induced myofibroblast activation in lung fibroblasts from IPF patients. Structural modeling suggests Ab-31 has a unique binding mode to αv integrins compared to existing antibodies.
The authors begin by laying out the rationale for targeting αv integrins in fibrosis. Five αv-containing integrins (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8) have been implicated in fibrosis through several mechanisms:
1. Transducing mechanical and biochemical signals from the fibrotic extracellular matrix
2. Activating latent TGFβ, a master regulator of fibrosis
3. Modulating fibroblast adhesion, migration, and growth
Previous studies have shown protective effects of genetic deletion or antibody inhibition of specific αv integrins like αvβ6 in animal models of lung fibrosis. However, the authors argue that the overlapping roles of multiple αv integrins suggest a pan-αv inhibitor may be most effective clinically. This hypothesis is supported by work showing depletion of all αv integrins in myofibroblasts protected against fibrosis in multiple organs. A recent genome-wide association study also found reduced αv gene expression correlated with improved lung function in over 400,000 individuals.
The authors first sought to further validate targeting αv integrins using the small molecule inhibitor MK-0429. This compound was originally developed as an αvβ3 antagonist but was found to potently inhibit multiple αv integrins. They show MK-0429 significantly reduced fibrosis progression in a mouse model of bleomycin-induced lung injury when administered after the initial inflammatory phase. This suggests the compound's effects are directly anti-fibrotic rather than just anti-inflammatory. The efficacy of MK-0429 provides additional preclinical support for pan-αv integrin inhibition as a therapeutic strategy. However, as a small molecule, it likely has limitations in terms of selectivity and pharmacokinetics. This motivated the search for antibody-based inhibitors.
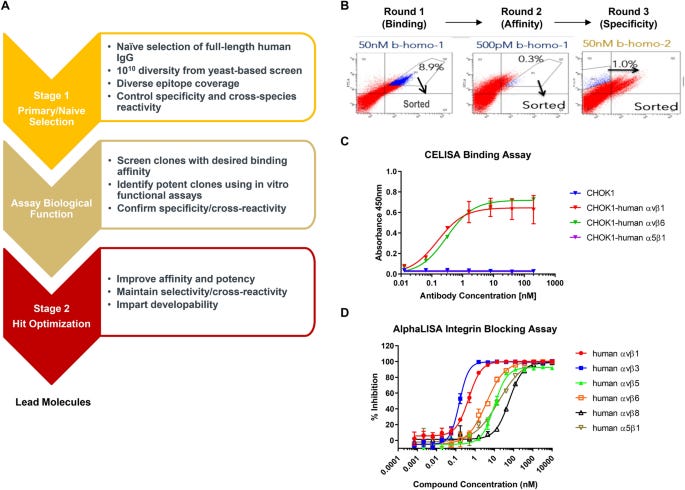
A key contribution of this work is the discovery of new monoclonal antibodies against αv integrins with unique properties. The authors used a yeast display platform to screen a large naïve human antibody library, selecting for binders that:
1. Recognized multiple αv integrins (αvβ1, αvβ3, αvβ5, αvβ6, αvβ8)
2. Cross-reacted with both human and mouse proteins
3. Did not bind the related α5β1 integrin
This yielded several antibodies meeting these criteria - a significant achievement given the high homology between αv integrins and lack of previously reported antibodies with human/mouse cross-reactivity. The authors focused on characterizing one lead antibody, Ab-31, which showed high potency across multiple functional assays: Blocking integrin-ligand interactions (IC50 < 10 nM for most αv integrins). Inhibiting integrin-mediated cell adhesion (IC50 1-6 nM). And suppressing integrin-dependent activation of latent TGFβ (IC50 3-20 nM)
Importantly, Ab-31 maintained potency against both human and mouse integrins in these assays. This is a valuable property for a tool compound, allowing its use in both human cellular studies and mouse in vivo models. Perhaps the most intriguing finding is Ab-31's potency in suppressing TGFβ-induced myofibroblast activation in lung fibroblasts derived from IPF patients. Using high-content imaging to quantify α-smooth muscle actin (αSMA) expression, a key marker of myofibroblast activation, the authors found Ab-31 potently inhibited TGFβ-induced αSMA expression (IC50 ~33 nM). This potency matched that of a TGFβ receptor inhibitor (IC50 ~56 nM). MK-0429 and another benchmarking αv antibody showed minimal effect
The strong activity of Ab-31 in this disease-relevant cellular assay is promising. The fact that it outperformed MK-0429 despite similar potency in biochemical assays suggests Ab-31 may have advantages in a cellular context. The authors speculate Ab-31 could preferentially recognize an active or disease-associated integrin conformation. If true, this might allow for improved efficacy and therapeutic index. However, this hypothesis requires further investigation.
To better understand Ab-31's properties, the authors used computational modeling to predict its binding mode to αv integrins. They compared this to crystal structures of two other αv antibodies in clinical development 17E6 (abituzumab) that binds exclusively to the αv subunit away from the ligand-binding site And LM609, binding at the interface of αv and β3 subunits near but not directly blocking the ligand-binding site.
In contrast, the model predicts Ab-31 binds at the α/β subunit interface and partially occludes the ligand-binding site. This unique mode of interaction could explain Ab-31's potency and pan-αv activity. While intriguing, it's important to note this is a computational prediction. Experimental structural studies will be needed to confirm Ab-31's binding mode and fully elucidate its mechanism of action.
This study advances the field of integrin-targeted anti-fibrotic therapies in several ways. It provides further preclinical validation for pan-αv integrin inhibition using the small molecule MK-0429. It introduces a set of novel antibodies with valuable properties for both research and therapeutic development. And demonstrates the potential for antibodies to have distinct and potentially advantageous properties compared to small molecule integrin inhibitors. Offering preliminary structural insights that could guide future antibody optimization efforts.
Several key questions and directions for future research emerge:
1. How does Ab-31 compare to more selective αv integrin antibodies (e.g. αvβ6-specific) in terms of efficacy and safety in vivo?
2. What is the basis for Ab-31's apparently superior activity in IPF patient fibroblasts compared to MK-0429? Is there truly preferential binding to a disease-associated integrin conformation?
3. Can Ab-31 or related antibodies be optimized for improved pharmacokinetics, tissue penetration, or other drug-like properties?
4. How do the effects of αv integrin inhibition compare or potentially synergize with other anti-fibrotic mechanisms being explored clinically (e.g. CTGF inhibition, recombinant pentraxin-2)?
The pursuit of integrin-targeted therapies has a long and complex history. While some notable successes exist (e.g. natalizumab for multiple sclerosis), many integrin inhibitors have failed in clinical trials due to lack of efficacy or unexpected toxicities. Fibrosis represents a promising new application for integrin-targeted drugs. However, several challenges remain. Inhibiting one integrin may lead to compensatory upregulation of others. Integrins can have different effects depending on cell type and disease stage. Complete inhibition of integrin function may disrupt important physiological processes.
The pan-αv approach explored in this study aims to address some of these challenges by targeting multiple integrins simultaneously. The discovery of antibodies like Ab-31 with unique properties opens new avenues for overcoming others. While many questions remain, this study lays a foundation for continued research and development. The cross-reactive antibodies described here will be valuable tools for preclinical studies. With further optimization, they or related molecules may advance into clinical testing, potentially offering new hope for patients with fibrotic diseases.