Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
Carmot Therapeutics is focused on developing novel therapeutics for metabolic diseases like obesity and diabetes. The company's proprietary drug discovery platform, Chemotype Evolution (CE), allows for the rapid discovery and optimization of small molecule drug candidates.
Obesity and diabetes are two of the most prevalent metabolic diseases globally, affecting over 750M people worldwide. These diseases significantly increase the risk of other severe health complications like cardiovascular disease, chronic kidney disease, and even death. Existing treatments have limitations in efficacy, tolerability, and convenience. There is a significant unmet need for improved therapies that can produce robust and sustained metabolic benefits for patients.
At the core of Carmot's drug discovery efforts is their Chemotype Evolution platform. This technology aims to rapidly navigate chemical space to identify novel small molecule drug candidates. The key steps of the platform are:
1. Identify a biological target of interest and a "bait" molecule that binds that target. The bait can be a known ligand, inhibitor, etc.
2. Derivatize the bait with reactive linkers to allow covalent bonding with fragment molecules.
3. Screen a proprietary library of fragment molecules against the target. Fragments that bind in proximity to the bait will form stable adducts.
4. Test the bait-fragment adducts for activity against the target using biochemical or biophysical assays.
5. Select the most active adducts, identify their structure, and synthesize follow-up compounds. The bait can also be altered.
6. Repeat the process using the new active compounds as baits to further optimize potency, selectivity, and drug-like properties.
This iterative screening approach allows for the rapid exploration of vast chemical space. Rather than screening a static library, the screening library is constantly changing as new bait-fragment combinations are generated. Even a modest fragment library of 1000 compounds could generate 1000^3 (1 billion) possible adducts after just 3 cycles. This enables remarkably efficient optimization of initial hit molecules.
The Chemotype Evolution platform shares conceptual similarities with other fragment-based screening approaches like SAR by NMR. However, a key difference is that CE starts with an initial bait compound rather than a blank slate. This allows the platform to rapidly focus on chemical space relevant to the biological target of interest. Overall, CE offers an innovative combination of fragment-based screening, covalent tethering, and iterative optimization.
Carmot is using the CE platform to develop a pipeline focused on treating obesity, type 2 diabetes (T2D), and type 1 diabetes (T1D). Their lead programs target peptide hormone receptors called GLP-1 and GIP, which regulate glucose metabolism and food intake.
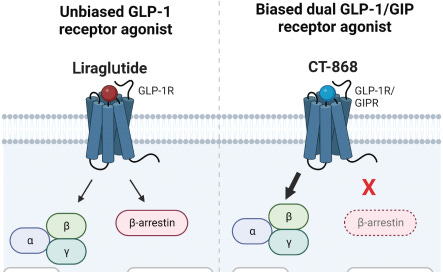
CT-388 is a long-acting GLP-1/GIP dual agonist designed for weekly subcutaneous injection. It is currently in Phase 1/2 development for obesity and T2D. In preclinical studies, CT-388 demonstrated biased signaling at both the GLP-1 and GIP receptors. This meant potent activation of beneficial cAMP signaling with minimal β-arrestin recruitment. These biased properties could lead to increased therapeutic activity and tolerability compared to unbiased agonists.
In June 2023, Carmot announced positive initial clinical data from the ongoing CT-388 Phase 1/2 trial. Statistically significant weight loss was seen across all doses after just 4 weeks of treatment. In the highest dose cohort, participants lost an average of 8.4% of their body weight. CT-388 also displayed good tolerability with mild GI-related side effects consistent with other GLP-1 therapies. These results provide clinical proof-of-concept for CT-388 and validation of the CE platform.
CT-996 is an oral small molecule GLP-1 agonist in Phase 1 development for obesity and T2D. Oral administration offers a major advantage in convenience for patients over injectable drugs. In October 2023, Carmot reported supportive preliminary Phase 1 data showing pharmacokinetics compatible with once-daily oral dosing. CT-996 was also well tolerated with mostly mild GI side effects. An efficacious oral GLP-1 therapy would be an important advance for diabetes and obesity treatment.
CT-868 is an injectable GLP-1/GIP dual agonist in development as an adjunct therapy to insulin for T1D. T1D patients have an impaired ability to produce insulin. CT-868 aims to improve glycemic control and reduce insulin needs in T1D. It is currently in Phase 2 trials. Early clinical data indicates CT-868 can lower blood glucose and HbA1c (a measure of glucose control). An adjunct therapy that reduces insulin requirements could meaningfully improve outcomes for T1D patients. In addition to these clinical programs, Carmot is also pursuing earlier stage research into new metabolic disease targets like PYY.
Carmot Therapeutics’ have to potential to play an important role in the metabolic disease space. Their innovative Chemotype Evolution platform allows for the rapid design and optimization of small molecule drug candidates. This technology has fueled a promising therapeutic pipeline, including novel GLP-1/GIP dual agonists with differentiated clinical profiles. Initial proof-of-concept data provides clinical validation of Carmot's approach. Carmot's patient-focused pipeline has strong potential to provide improved treatment options for patients with obesity, T2D, and T1D.
In late 2023, Carmot was acquired by Roche for $2.7B upfront plus up to $400 million in milestone payments. The acquisition gives Roche access to Carmot's pipeline and expertise in metabolic biology to develop new therapies. The deal is expected to close in Q1 2024. The acquisition reflects Roche's strategy to grow through targeted deals to enhance its pipeline in specific disease areas like obesity and diabetes.