Yale is a burgeoning source of great life sciences companies. With growing support from the university and the surrounding area, inventors from Yale are in a position to create exciting, new businesses from Arvinas to Simcha.
Crews Lab
Using chemical biology to understand cell biology.Recent
Defining the rules of proteolysis targeting chimeras (PROTAC) design - https://www.sciencedirect.com/science/article/abs/pii/S1367593118301522?via%3Dihub
Viewpoint on the power of studying regenerative organisms - https://www.nature.com/articles/d41586-017-09008-4
Past
Review on the power of small molecules to control intracellular protein levels - https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201307761- important as a research tool, but even more important to pursued undrugged targets
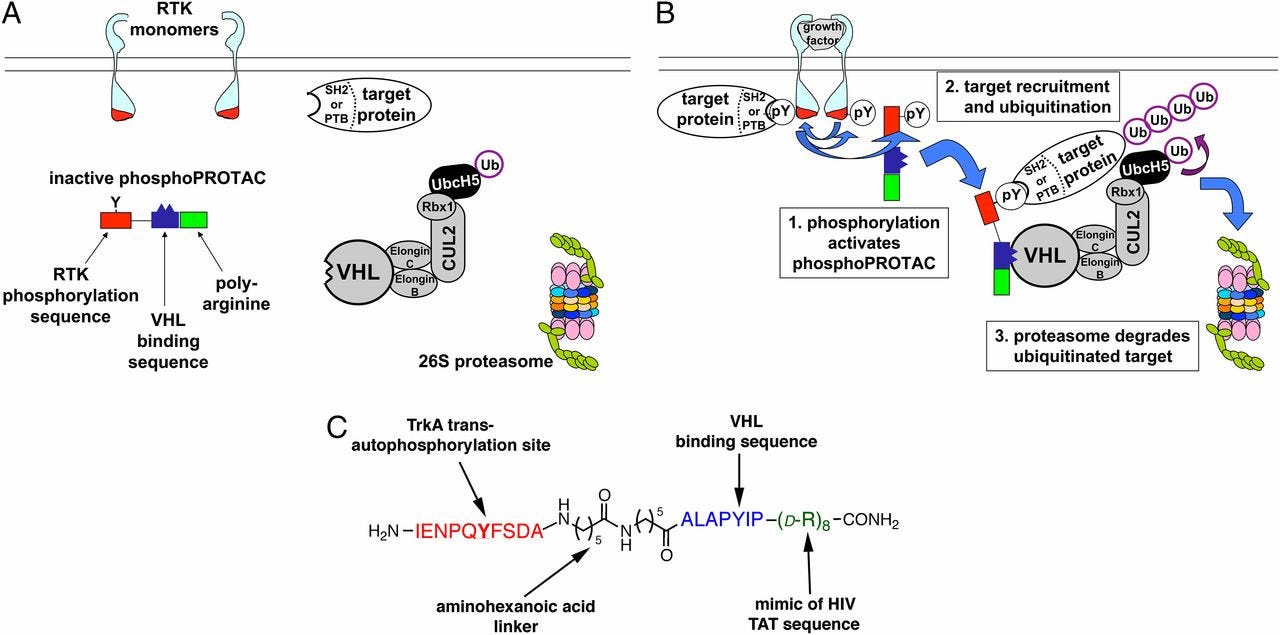
Inventing PROTACs that are coupled to kinase activity - https://www.pnas.org/content/110/22/8942.long- where the PROTAC is activated upon phosphorylation activating specific pathways:
Steitz Lab
Studying and engineering RNA machinery.Recent
Review from the world-expert on how coral noncoding RNAs affect the host - https://www.annualreviews.org/doi/abs/10.1146/annurev-virology-092818-015811
Past
Overview of computational methods to discover and characterize noncoding RNAs in viruses - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684774/- important to begin to make this class an attractive clinical target.
Campbell Lab
Computational and experimental analysis of heart disease.Recent
Review of methods to use human induced pluripotent stem cells (iPSCs) - https://physoc.onlinelibrary.wiley.com/doi/pdf/10.1113/JP276753- to establish genotype/phenotype relationships for cardiomyopathy:

Review of work done to model cardiomyopathy - https://www.jmmc-online.com/article/S0022-2828(18)30975-1/fulltext
Past
Review of computational methods to predict familial hypertrophic cardiomyopathy from biophysical/genomic data - https://royalsocietypublishing.org/doi/pdf/10.1098/rsif.2011.0184
Using computational models of ventricular myocytes to discover to mechanisms driving excitation–contraction coupling in the heart - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2556206/- important proof-of-concept to show the value of computational analysis to clinically manage cardiomyopathy.
Integrating genetics and biophysical data to predict how myofilaments affect cardiomyopathy - https://www.frontiersin.org/articles/10.3389/fphys.2016.00473/full
Isaacs Lab
High-throughput genome engineering.Recent
Designing a genetically encoded method to incorporate an unnatural amino acid into polypeptides for selective reactions with drugs - https://isaacslab.yale.edu/sites/default/files/costa_2019_acs.nanolett.8b03837.pdf- important to create drug delivery systems with precise control. Valitor is doing great work to pursue a similar problem:

Overview of new methods to expand genetic codes - https://isaacslab.yale.edu/sites/default/files/arranz-gibert_cocb2018.pdf
Past
An iconic paper to establish the method to genetically recode an organism - http://isaacslab.yale.edu/sites/default/files/lajoie_isaacs_science13_0.pdf- forming the basis of GRO Biosciences and 64-X:

With the Jewett lab, another great inventor, engineering translation to improve multiplep-azido-phenylalanine (non-canonical amino acid) incorporation - https://isaacslab.yale.edu/sites/default/files/gan_et_al-2017-biotechnology_and_bioengineering.pdf
Rinehart Lab
Decoding the human phosphoproteome.Recent
Recoding E. coliusing technology pioneered by the Isaacs lab to mimic the human serine phosphoproteome - https://isaacslab.yale.edu/sites/default/files/barber_nbt_2018.pdf- important to discover molecules that interact with phosphorylated serines to drug. Also, very valuable to create post-translational modification (PTM) libraries with physiological relevance:
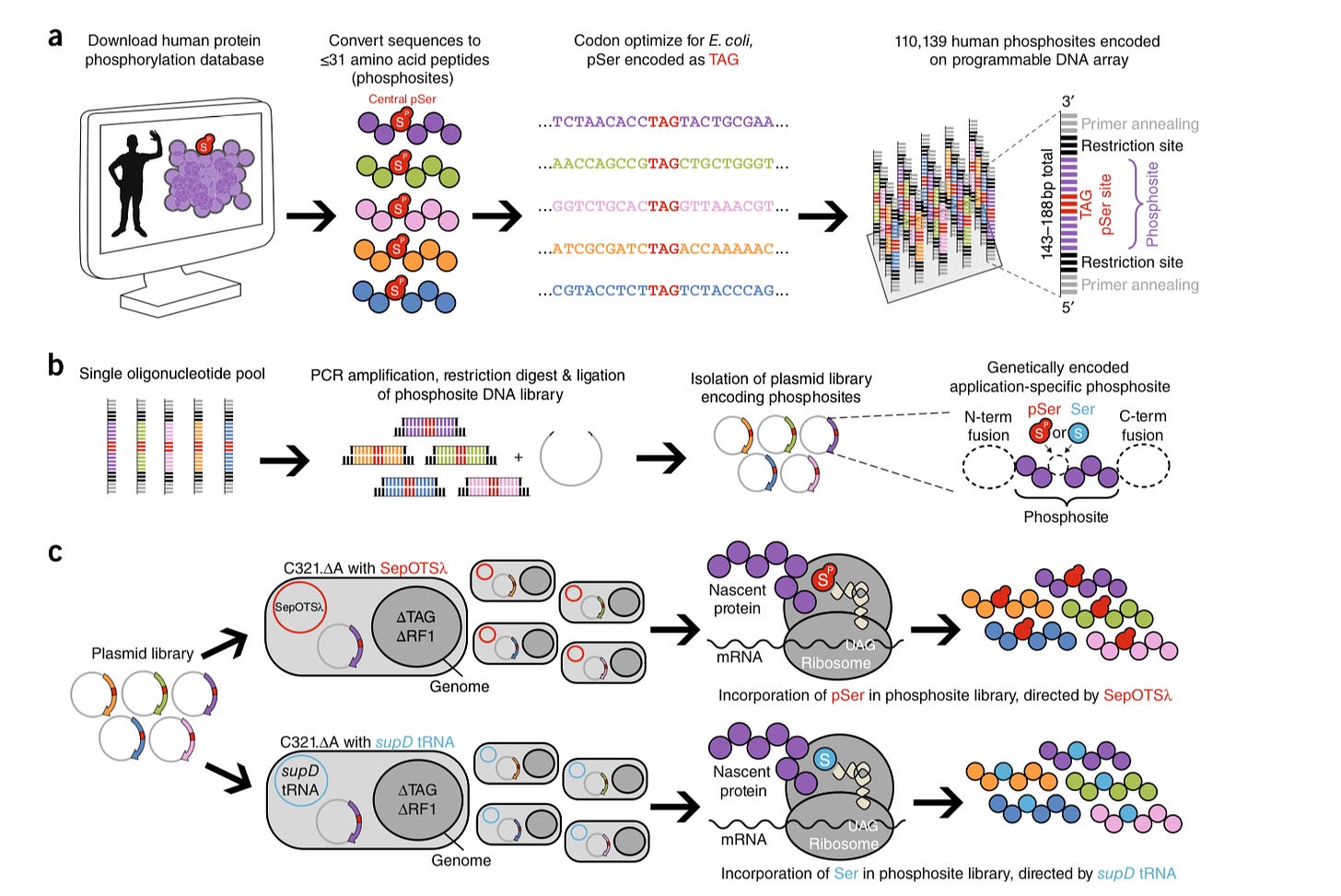
Invented SERIOHL-KILR to profile and identify kinase substrates -https://pubs.acs.org/doi/10.1021/acs.biochem.8b00410- using E. coli-derived human peptides (with serines) and liquid chromatography and tandem mass spectrometry(LC-MS/MS) to measure in vitrokinase activity against the peptides:

Ring Lab
Engineer immune receptors using combinatorial biology.Past
Evolving interleukin-2, a T- and NK-cell growth factor, to be specific toward IL-2Rβ independent of CD25 to imbue specificity to create super-2, an IL-2 superkine - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3338870/- promising approach to engineer interleukins for immunotherapies (Simcha, Neoleukin), but competition from other modalities could be an issue given proleukin revenue is in the $10Ms.
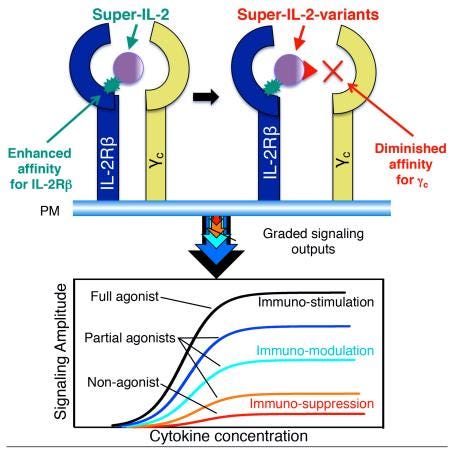
Building on top of the superfine work, to create IL-2 variants that act as clamps - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4560365/- enabling fine-tuning of the signal amplitude:
Using yeast display to evolve a protein therapeutic (14 kDa, about 10x small than a monoclonal antibody) against PD-1 -https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4664306/- showing the ability to design non-antibody proteins against high-value targets where size of the drug could occlude target occupancy and reduce efficacy.
Joshi Lab
Investigate immune cell interactions within developing tumors.Recent
Inventing a mouse model (NINJA) to study tissue-specific peripheral toleranceimportant for T- and B-cells -https://www.jimmunol.org/content/200/1_Supplement/47.6
Viewpoint on T-regulatory cells (Treg) plasticity and how it enables fine-tuned activity depending on a microenvironment - https://www.nature.com/articles/s41590-019-0389-y
Past
With the legendary Tyler Jacks, overview of how tumors actually activate Tregs to suppress the immune system that would attack the tumor - https://science.sciencemag.org/content/339/6124/1160.long
Important immunology paper to characterize tertiary lymphoid structures (TA-TLSs) - https://static1.squarespace.com/static/57e329372e69cffe309f906f/t/59c695b5cd39c354a175a7a0/1506186684556/1-s2.0-S1074761315003180-main-3.pdf- discovering that TA-TLSs recruit T-cells in particular Tregs for lung cancer in order to suppress anti-tumor activity.
Schatz Lab
Generating a diverse and effective repertoire of antibodies during an immune response.Recent
Evolution has been a great inventor; analysis of V(D)J recombination and RAG 1/2 evolution - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5459667/- in order to understand the origins of how diversity is generated for antibodies (Ab) and T-cell receptors (TCR).
Using cryo-electron microscopy (cryo-EM) of a relatives of the RAG proteins to understand how they evolved from acting as a transposase (moving across a genome) to a recombinase (recombining genes) and gaining specificity to generate Ab and TCR diversity - https://www.nature.com/articles/s41586-019-1093-7
Past
Discovering that somatic hypermutation, which generates point mutations in B-cells to create diverse antibodies is influenced by antibody enhancers (activating genetic regions) to create a strong positive feedback loop to maximize Ab diversity - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972084/
Rothman Lab
Studying intracellular transport.Recent
Rothman along with Randy Schekman (UC Berkeley) and Thomas Südhof (Stanford) won the Nobel Prize in 2013 for Physiology or Medicine for discovering the “machinery regulating vesicle traffic, a major transport system in [cells].” Incredible work important for biologics manufacturing helping generate $100Bs in value. Nobel Lecture on the topic; an aside, reading these lectures in general are a great way to know what is important in life sciences - https://www.nobelprize.org/uploads/2018/06/rothman-lecture-1.pdf
Offering a framework to become a great inventor - https://febs.onlinelibrary.wiley.com/doi/full/10.1002/1873-3468.12960
Framing that software is helping biology make the transition from analog to digital instead of automating it (conventional but wrong wisdom) - https://febs.onlinelibrary.wiley.com/doi/full/10.1002/1873-3468.13308
Using cryo‐electron tomography to discover the precise arrangement of the machinery required for exocytosis (active transport out of the cell) -https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6353562/
Past
Inventing single-stranded DNA conjugated with lipids to regulator specific SNAREs (important for membrane fusion) - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4888371/- important as an orthogonal tool for research and bringing two selected membranes together.
With Schekman, reviewing the field of protein folding - https://www.cell.com/cell/fulltext/S0092-8674(11)01011-7
Miller-Jensen Lab
Studying how cell signaling and cell-cell communication regulate immune cells.Recent
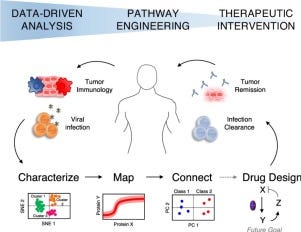
Overview of systems immunology - https://www.sciencedirect.com/science/article/abs/pii/S0958166918300119?via%3Dihub- to use multi-dimensional (many sources) and high-content (well characterized) to connect the state of the immune system to the state of the disease. Important work to use host-based reading to develop new medicines:

Past
Mapping out the toolbox to characterize signaling pathway diversity at the single-cell level - https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(16)00043-3
Great work on analyzing single-cell cytokine secretion - https://stke.sciencemag.org/content/8/381/ra59- to discover that a decrease of IL-10 in a specific cell population, monocyte-derived macrophages, leading to a cascade of events and inhibition of inflammation. Important for showing the power of the method (single-cell, perturbation) to validate new targets.


