Axial partners with great inventors creating unique business models. Profiling exciting life sciences companies at the earliest stages is important. Rather than talk about their work specifically conveying the opportunity set is more important where the company and others in the field will bring to market currently confidential inventions to more people.
Synthetic biology
Synthetic biology brings engineering principles to biology with the key principles:
Standardization of parts – makes screening and discovery reproducible and scalable
Coupling screening to the synthesis and assembly of DNA
Modularity of parts between multiple chassises
More predictable outcomes – as more data is collected through screens various combinations of components will be discovered not to work together
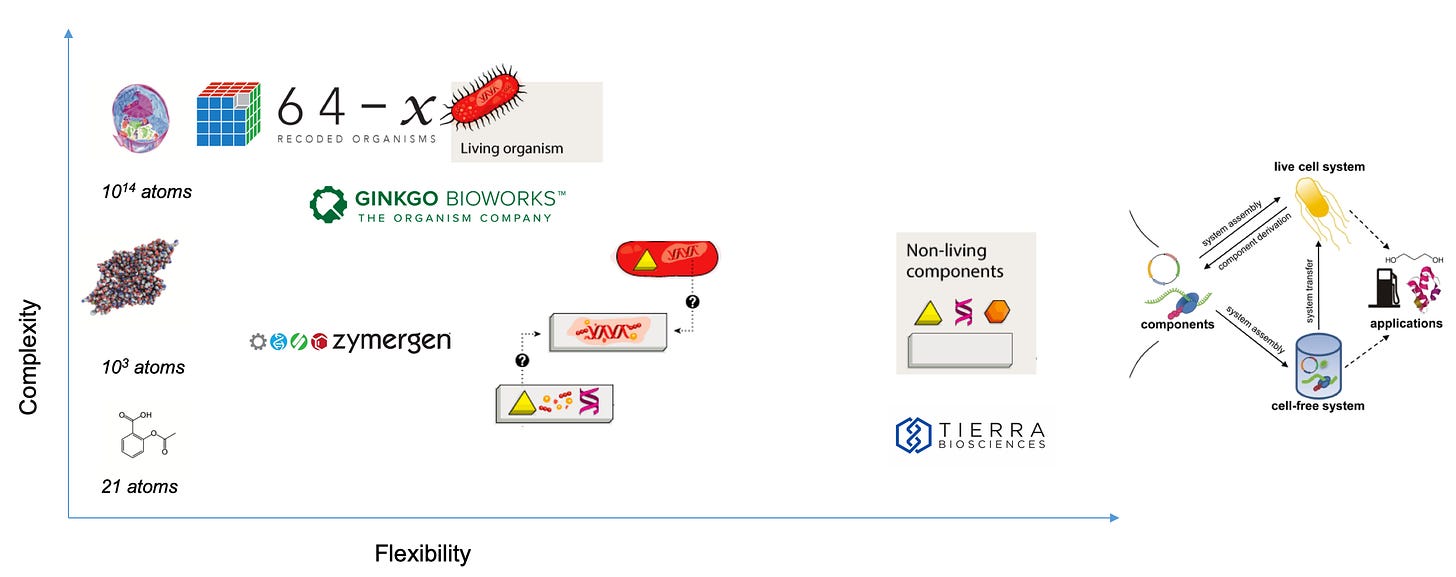
Companies like Ginkgo Bioworks and Zymergen are leaders in the field on the backs of many pioneers with companies like Lygos, Arzeda, Bolt Threads, Aether, Checkerspot, and Visolis doing exciting work:
Just as recombinant DNA technology created a whole new fields generating $100Bs in value, the ability to use synthetic biology to generate large libraries of genes to design new functional, biological constructs is incredibly powerful. Tools from synthetic biology allow a spectrum of flexibility/complexity capabilities to engineer large systems with many biological parts. The design process in synthetic biology is relying less on traditional cloning and more on high-throughput assembly, synthesis, and editing. As a result, the process is now limited by the ability to simulate circuits, analyze data, and create unique, functional constructs:
Parts characterization
Interoperability
Diversity of designs
Selection
Ultimately, this potential could lead to the production of almost any molecule imaginable on much shorter timescales than ever before relying on an ever expanding toolbox:
Recombinant DNA/cloning
Culturing
Directed evolution
Sequencing
Synthesis
High-throughput screening (i.e. NMR, microarrays, automation)
Computational modeling
CRISPR (likely the key driver for much of the progress in the field over the last 5 years)
As systems become larger and more complex, their interactions with endogenous systems have become more acute. Bioengineers will need to develop methods to account for burdens that synthetic systems place on their hosts:
CRISPR enables synthetic biologists to take a more holistic engineering approach by modifying synthetic circuits and the host genome with relative ease
Synthesizing whole genomes will require a 1000x reduction in the cost of synthesis (low probability in 5 years)
Given this, each past technology cycle has relies on a set of new tools:
Mechanical – machine tools, nuts, and bolts
Transportation – trains, cars, roads
Chemical – large scale production
Digital – hardware, communication protocols, data format
For synthetic biology, the field has components such as chassis, operons, sequencing, ligation with the field is composed of three main layers: software, actuators, biology. Business models can be in a part of a layer or can be full-stack integrating where needed. Full-stack companies have advantages of maintaining interoperability and have improved economics depending on the end product. Layer companies have advantages of more flexibility and portability. With the spectrum of models pursuing opportunities around tools, therapeutics, drug delivery systems, agriculture, specialty chemicals, bio-manufacturing, commodity chemicals, and biomaterials. Many industries (i.e. transportation, chemical) after the digital era hit inflection points with regards to scale and efficiency once they connected software to their physical systems. Similarly, synthetic biology will probably accelerate as software becomes more connected to biology.
Have a nice day.