Axial partners with great inventors creating unique business models. Profiling exciting life sciences companies at the earliest stages is important. Rather than talk about their work specifically conveying the opportunity set is more important where the company and others in the field will bring to market currently confidential inventions to more people.
Cancer vaccines
Cancer vaccines offer a new way to treat cancer through prevention instead of intervention. However, the last 10-20 years, the results from the field have been lackluster - some parts technology, drug pricing, and incentives. New tools have emerged to make cancer vaccine development easier, but the healthcare system is still tackling problems of prices and measuring outcomes.
Many exciting companies have emerged over the last decade to pursue their own approach to develop and commercialize a new cancer vaccine: Gritstone, Neon Therapeutics, Caperna (Moderna), Immune Design (Merck), HelixNano, BioNTech, Guardant Health, and CureVac.
Overview
Cancer vaccines stimulate the body’s immune system to recognize tumor cells as non-self to induce the immune system to clear the cancer cells. Cancer vaccines may be an entire cell or contain specific peptide/protein antigens. The design process is focused on dendritic cells (DC), which are the master coordinators of the immune system. DCs take up an antigen and load itself with it traveling to the lymph nodes to activate T-cells. Other molecules called adjuvants (i.e. viral vectors, cytokines) can be added with a vaccine to induce DCs to execute this process more efficiently. Once T-cells are activated, they go on the hunt for their targets. With the success of checkpoint inhibitors, cancer vaccines can build on top of them in order to improve response rates and move toward proactively preventing cancer.
What’s changed?
When compared to checkpoint inhibitors and other immuno-oncology (IO) drugs, cancer vaccines have been a failure with only two approved cancers approved by the FDA with minimal impact on patients. One of the therapies, Provenge, had pricing problems, which seem non-important when compared to the prices of CAR-T and gene therapies, but most of the issues limiting cancer vaccines are technical:
Limited number of truly foreign antigens (neoantigens) expressed by tumor cells combined with chronic antigen exposure leading to lower efficacy rates overtime
Immunosuppressive tumor microenvironments (TME) limiting efficacy against solid tumors
Lack of understanding what drives a strong and sustained T-cell response in humans (getting better each year but still a lot of mechanisms to map out)
These problems are large opportunities for new companies to overcome and bring to patients an effective cancer vaccine. The super-exponential decrease in the cost of sequencing has enabled to the identification and validation of neoantigens. Over the last decade, the influx of neoantigens has helped overcome past suboptimal design to create vaccines that can target a wider range of antigens. The TME is still a very hard problem to overcome for any oncology drug but improving adjuvants have shown improved cancer vaccine activity.
Examples of useful targets for cancer vaccines are:
Cancer–testis antigens: BAGE, GAGE, MAGE, NY-ESO-1
Differentiation antigens: CEA, gp100, Melan-A, PSA, tyrosinase
Over-expressed antigens: HER2, hTERT, p53, survivin
Oncogene‐associated antigens: β‐catenin, HSP70, KRAS
Glycans: GM2, MUC1
Getting the right combination of targets will likely make or break the success of any cancer vaccine not only for efficacy but feasibility of use. To go from diagnosis to a personalized vaccine takes months and without a scalable manufacturing solution, the timing and cost may be incompatible with cancer. As a result, discovering driver neoantigens that directly influence tumor growth, as opposed to passenger mutations, is incredibly important to develop broadly used vaccines. Picking the right combination of a driver and passenger targets (a place software will help), will help a drug deal with tumor heterogeneity and create a durable immune response.
With cheaper sequencing and checkpoint inhibitors, cancer vaccines are more viable than in the past. These recent advances have allowed phase I clinical trials of neoantigen vaccines to start. Importantly, most of the previous cancer vaccine studies were done on patients with advanced cancers, which lowered efficacy with survival extension in the months (i.e. Avax, Geron, Dendreon, Introgen). With better diagnostics (i.e. Guardant Health), there is a shift toward testing vaccines on patients with less developed cancers reducing suppression of the therapy. Ultimately, there is an enormous but still untested potential of vaccines for cancer prevention instead of conventional cancer therapy. It is more effective to constantly surveil for cancer in order to prevent its development.
Measuring a response
During development, measuring patient response of cancer vaccines has been difficult. There are a few different ways of measuring T-cell responses:
Quantity - sheer number of cells
Quality - activity of cells; some just are too exhausted to function properly
Breadth - diversity of cell types
Avidity (most important feature) - strength with which a T-cells binds an antigen
So for most cancer vaccines have not evoked sufficient quantities of antigen-specific T-cells - ideally, a vaccine would generate ~10% of circulating T-cells that are specific to a set of antigens, but most cancer vaccines yield somewhere around 0.1%. There have been improvements, but designing platforms to make immune system measuring seamless ought to help create cancer vaccines that go from 0.1% to 10% generation rates.
Using the immunogenic cell death pathway
The immunogenic cell death (ICD) pathwayis a unique mechanism of action (MoA) to solve the historical three challenges for cancer vaccines. With checkpoint inhibitors, the ICD pathway can be invaluable to reduce tumor burden, increase antigen accessibility, and induce a strong immunological response.
To engineer something, one has to understand its history and development. ICD can be very useful as a secondary mechanism to induce a robust immune response, but like any event in biology there are limitations due to complexity. For ICD, subversion strategies are the largest one. The second is controlling the response and side-effects – right now, the trade-off is between universality and systemic inflammatory responses.
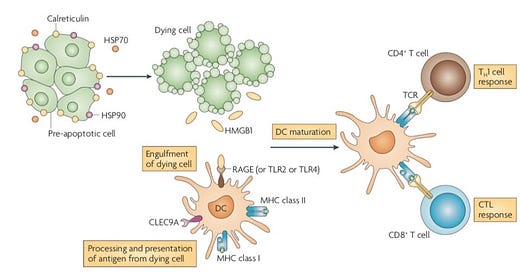
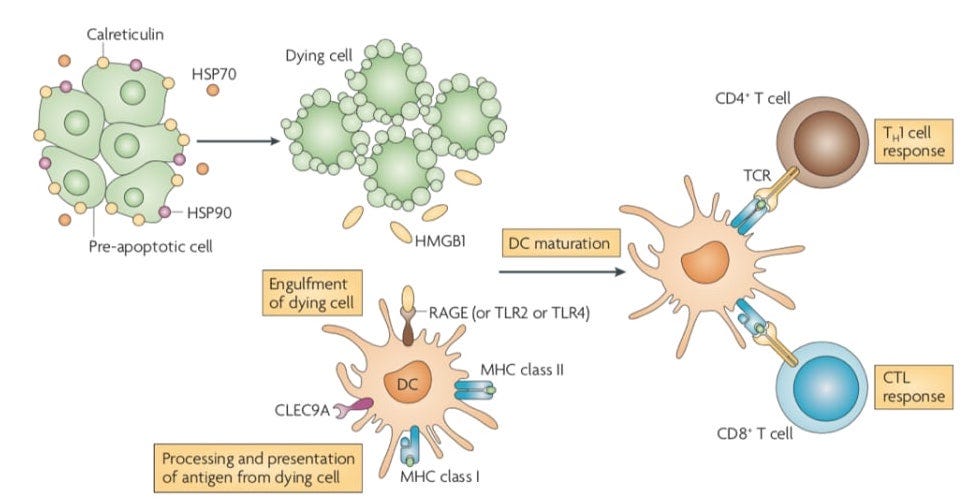
ICD can be generalized by a set of patterns (image below):
Release of ATP and chaperone proteins
Release of HMGB1 after apoptosis, which are recognized by TLRs
Presence of calreticulin on the membrane
Interferon and interleukin production
The human body has evolved ways to turn over cells in the tissues in steady state with a trillion cells dying each week. As more complex tissue was created, a more complex immune system co-evolved. As a result, there is a strong linkage between cell death (the spectrum between apoptosis and necrosis – not a binary response) and vertebrate immune systems to identify self from non-self. Cell death is a spectrum with apoptosis the controlled version and necrosis the relatively uncontrolled one with ICD closer to necrosis but interestingly early studies have found consistent patterns in ICD (list above). Apoptosis is tolerated by dendritic cells (DCs) and mostly is not autoreactive. Whereas, necrosis releases danger signals inducing an immunogenic and inflammatory response. Then there this is everything between the two, which can be generalized into a combination of signals the immune system (adaptive and innate) recognizes as exogenous (bad – ATP, oxidative species) and endogenous (good – CD31, CD47). Therefore, therapeutically-relevant induced ICD most likely has a unique set of patterns that need to be distinguished from a good signal in order to create the strongest response possible.
Over time, an invader (bacteria or cancer) can develop strategies to evade immuno-surveillance. The primordial version of the ICD and the vertebrae immune system in general most likely protected against microbial infection. With this view, one could see the multiple strategies microbes and viruses developed to subvert the ICD response - the selective pressure to evade ICD was high so multiple inventions must have been created to evade infection. Most of the literature shows that bacteria and viruses are focused on augmenting production of adjuvants from the host but not modifying antigens (genes).
From this, maximizing adjuvant production, emission, and sensing could be the most important driver for success. From evolutionary analysis, if adjuvant maximization is the pathway modified to evade ICD then it must be the most effective mechanism-of-action. What is the most effective means to maximize adjuvant production in cancer cells not normal ones? Maybe upregulating the UPR, cell death signaling, autophagy in cancer cells? For ICD to induce a durable response to remove and surveil cancer, the correct and maximum amount of molecular patterns need to be designed to activate a robust immune response that reduces the probability of long-term tolerance.
Questions:
For each unique ICD response, what signals are specific?
What are the commonalities between ICD responses?
Which patterns on interchangeable? Which ones are not?
What links autophagy and cell recycling to ICD signaling?
By what magnitude do checkpoint inhibitors enhance ICD by unleashing the immune system? Negative side-effects?
Which patterns act at short-range and which patterns act at a long-range? Long-range stressors are a more important problem.
Are there genetic signals for ICD response? Maybe epigenetic?
Source: Nature
The promise of cancer vaccines is exciting but a lot of work is left due to the high number of strategies cancer uses to evade and suppress the immune system from expanding myeloid-derived suppressor cells (MDSCs), changing host metabolism, and perturbing cytokine networks. Once these problems are solved, the ability to map out an individual’s genetic history and calculate their risk for cancer could enable life-long cancer-specific immune surveillance.
Have a nice day.