Axial - Innovative Targeting Solutions
Surveying great inventors and businesses
Innovative Targeting Solutions (ITS) is a protein engineering company. On the other side of Vancouver from AbCellera, ITS is centered around a breakthrough technology to generate antibody diversity. Over 10 year in the making, ITS was founded by Michael Gallo, who previously led research on antibody generation at Amgen and Abgenix. With a business model similar to Adimab and AbCellera pursuing the same markets, what makes ITS an interesting case study is the uniqueness of their technology - HuTARG - and how the business used this as the basis to parter with some of the world’s largest drug developers. Given the value in ITS’ technology, the company serves as a useful starting point to identify new technical opportunities in antibody discovery and protein design in general.
Source: ITS
Key findings
Antibodies have a set of problems where a company can build up a technical moat around: target solubility, potency maturation, tolerance, immunogenicity, rare specificities, diversity (i.e. CDRs).
ITS’ HuTARG platform’s ability to engineer V(D)J recombination and scale up the process, enables the simultaneous discovery of specific antibodies and selection on stability, expression and solubility.
Due to the power of HuTARG, ITS likely has the largest functional (by the six main metrics) library of antibodies. The library not only consists of antibodies and receptors, but all of the human V, D, and J segments.
Life sciences companies initially tend to focus on solving technical problems as they are pressing; however, the ability to think hard about the business model and to design a unique one can generate enormous amounts of leverage with regards to growth and can be the difference between success and outsized success.
Technology
Screening
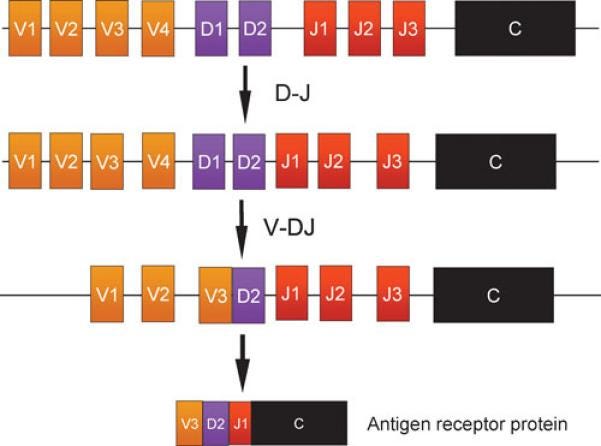
ITS’ HuTARG platform is an in vitro system recapitulating V(D)J recombination (nature’s most powerful diversification system; image below) through RAG1/RAG2 (fundamental work pioneered by the Baltimore Lab). Over 50 years ago, the realization that B-cells generate a diverse repertoire of antibodies led to intense research to understand the mechanism. Ultimately discovering that the exons encoding the antigen binding regions (for both antibodies and T-cell receptors) are assembled from V (variable), D (diversity), and J (joining) genetic segments by chromosomal breakages and rejoining in developing lymphocytes. This process is called V(D)J recombination:
Choosing a pair of genetic segments
Introducing double-stranded breaks next to each segment
Removes or inverts the intervening DNA
Ligates the segments together
Each pairwise reactions occurs orderly despite the potential damaging breaking of chromosomal DNA. The D segment joins the J segment before the V segment in a combinatorial manner. Diversity is generated by the potential combinations of different V,D,J segments and amplified by the variability at the junctions between the segments (inter and intra) - leading to an almost limitless repertoire where each individuals is nearly unique (99%) when compared to someone else.
Given the chromosomal DNA is broken millions of times over the lifespan of an organism, a high degree of fidelity in the V(D)J process must be ensured. The core discovery of this process were the recombination activating gene 1 (RAG1) and RAG2 proteins, which ensure that segments combine in the proper order. The RAG proteins interact with a small region of highly active chromatin in each segment region - creating recombination centers regulation the ligation of segments. RAG2 interacts with a trimethylated histone H3 lysine 4 (H3K4me3) and recruits RAG1 to centers. This is where the RAG proteins bind, cut, and resolve genomic DNA at recombination signal sequences (RSS) next to V,D,J segments:
Source: ASM
Source: ITS
This enables creating billions of diversified, unique fully human antibodies and even T-cell receptor therapeutics:
Source: ITS
Antibodies have a set of problems where a company can build up a technical moat around:
Target solubility
Potency maturation
Tolerance
Immunogenicity
Rare specificities
Diversity (i.e. CDRs)
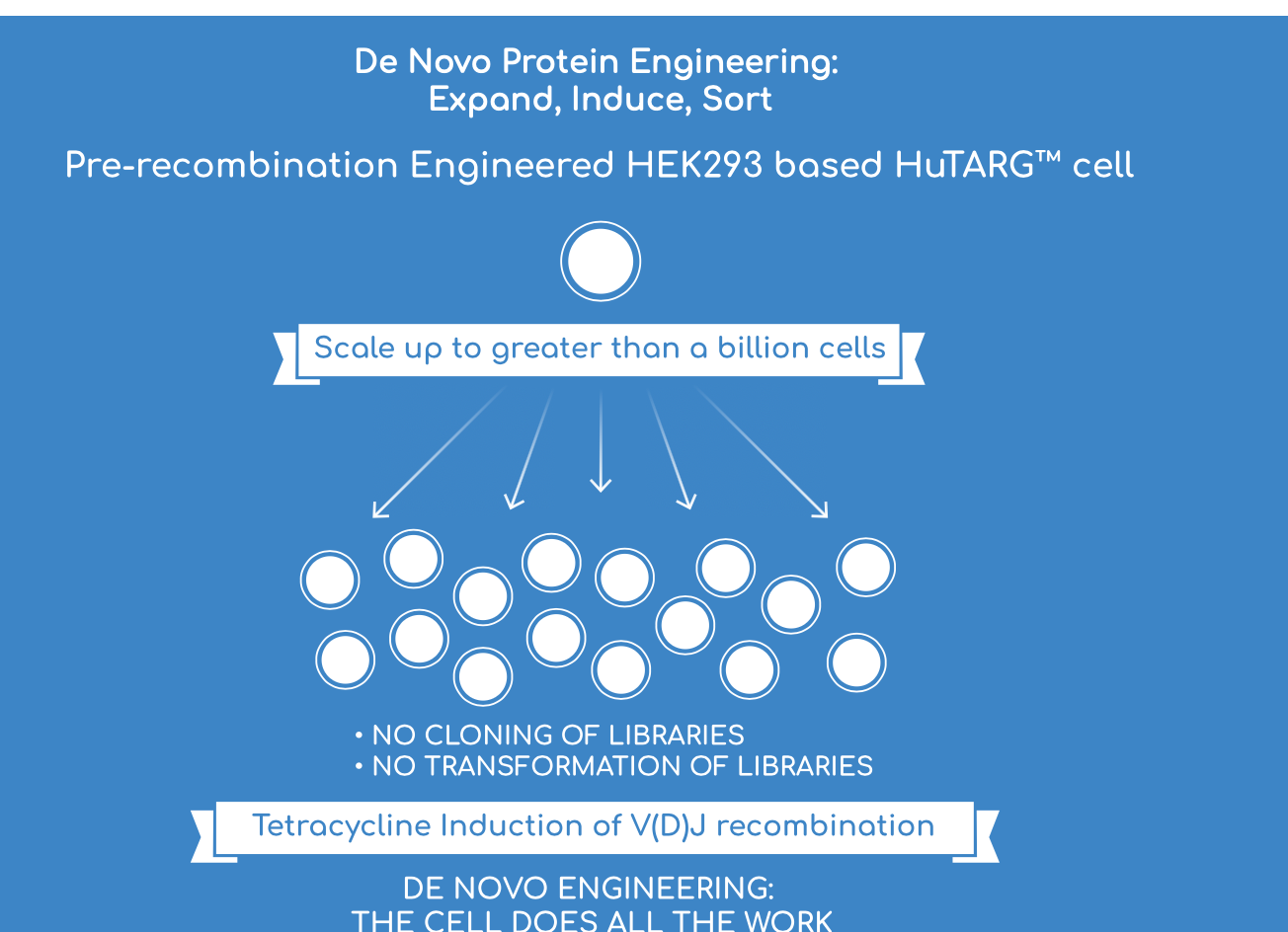
HuTARG recreates V(D)J in vitro to do generate billions of diverse antibody CDRs. By doing a reaction in vitro, the platform enables a functional away of full-length receptor in their native conformation on the cell surface. Combined with a high-throughput method (billions of cells), HuTARG assesses a large repertoire (billions of variants) in a realistic fashion. This method enables the creation of a large, functional library as it requires no cloning/transformation. In the system, complex screens (receptor neutralization, species cross-reactivity) can be designed to pull down antibodies with unique functions. The functional aspect helps improve ITS’ ability to discover a molecule with desirable features around target solubility, potency maturation, tolerance, and immunogenicity. The ability to optimize CDRs gives ITS the unique ability to do screen for affinity maturation in parallel to discovery. The ability to generate diverse libraries can potential discover molecules with rare specificities. HuTARG really shines on its ability to create a large functional library. Where Distributed Bio has the largest library of human antibodies, ITS has the unique ability to diversity and functionalize antibodies at an unprecedented scale.
The ability to screen billions of antibodies directly for function creates the opportunity to discover agonists/antagonists for currently intractable targets. Overall, HuTARG relies on three principles to build a valuable moat around diversification -expand, induce, and sort:
Source: ITS
Source: ITS
Source: ITS
In HuTARG, a cell expressing a non-agonist antibody will not activate cell surface reporter marker whereas cells expressing an agonist antibody will activate the reporter allowing isolation of the cell with the agonist (driven by cAMP reporter genes). Tetracycline is used to induce recombination to generates billions of variants. The throughput of the platform from a back-of-the-envelope math is:
300 ml = around 1 billion cells given 3.0 x 10^6 cells per ml
1 billion cells = 1 day of FACS sorting
3000 ml is approximately 10 billion cells = 10 days of FACS sorting
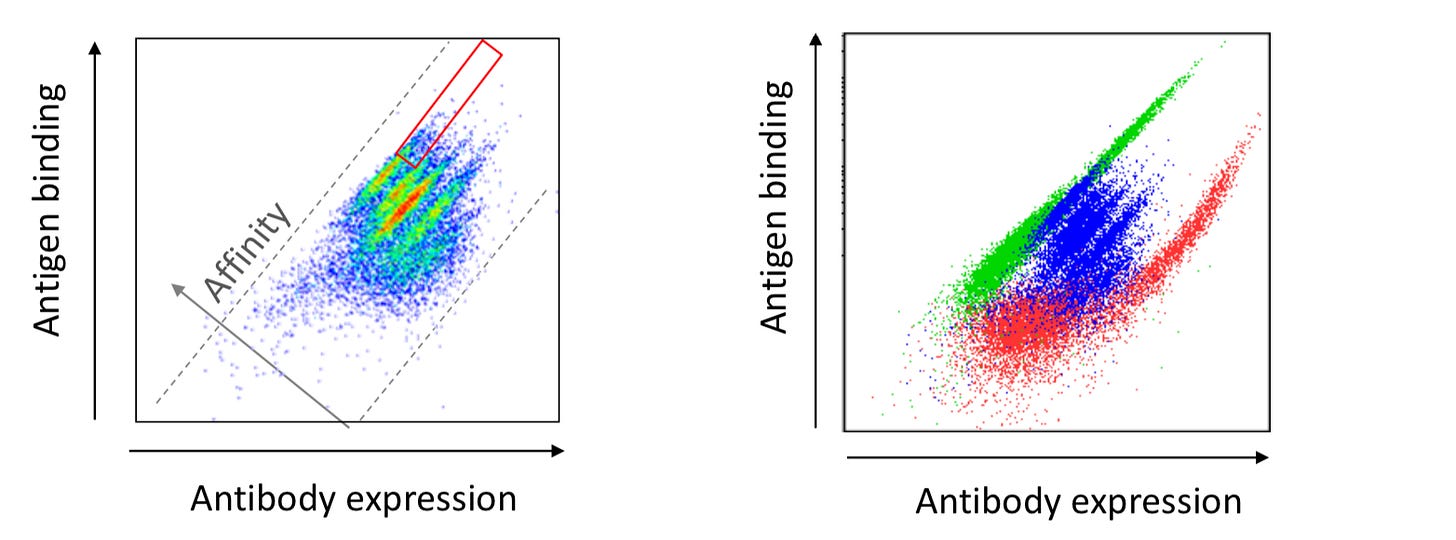
“To generate diversity, HuTARG is analogous to the in vivo system but directs mutations only to the CDRs keeping the framework regions intact within the antibody. In vivo, affinity maturation occurs in secondary lymphoid organs where low affinity fully germline antibodies are matured via somatic hypermutation.” Unlike the in vivo system, “framework regions in HuTARG are maintained in a germline configuration and saturating mutagenesis of the CDRs are probed in a single round of screening. The technology can be applied to mature antibody pools allowing one to generate panels of high affinity antibodies in parallel.” Fluorescence activated cell sorting (FACS) is used to identify and isolate antibodies with improved affinities. For HuTARG, the FACS sorting in the mammalian environment allows for the simultaneous selection of affinity and good manufacturability properties (i.e. expression). Cells with the highest ratio of antigen binding to antibody expression are isolated by flow cytometry - where these genes encoding highest affinity antibodies are cloned, sequenced, and analyzed. Along the way, CDR optimization can be performed on all CDRs (heavy chain and light chain) sequentially or in parallel. For bi-specific development, this optimization can be flipped to start with light chains instead of heavy ones.
Through RAG proteins and TdT, the HuTARG platform can tune deletions/insertion to improve final length of the antibodies, affinity maturation, and expression. With billions of variants screened for affinity and expression, the platform generates 100x-1000x improvement after a single round - red: antibody expression, green: high affinity variant isolated from library by flow cytometry, and blue: original antibody:
Source: ITS
Ultimately, HuTARG allows the creation of libraries using all the V- D- and J- genes. Importantly, without the need of complex regulatory regions required for transgenic technologies and without immune tolerance that would delete large portions of the immune repertoire and potential diversity. The systems allows the “isolation of specific antibodies and simultaneously, because it is a mammalian system, the selection fully-human antibodies with superior stability, expression and solubility.”
Library
Due to the power of HuTARG, ITS likely has the largest functional (by the six main metrics) library of antibodies. The library not only consists of antibodies and receptors, but all of the human V, D, and J segments. This supports the generation of a library with minimal homology within it to pursue rare specificities, which is determined by a limited number of amino acids that drive targeting of epitopes and MHC-peptide complexes
The HuTARG platform “enables the simultaneous maturation of pools of germline antibodies but can also be applied to lead optimization of individual candidates. As a result, the process of affinity optimization in mammalian cell lines removes the need for extensive back mutagenesis of antibody framework mutations as only high affinity antibodies with intact germline frameworks are generated.” This engineering process uses single-cell functional screens (over 1 billion cells) across large repertoires (over 1 billion unique CDRs) to identify leads with high affinity and manufacturability properties simultaneously.
Business model
With its technical breakthrough, ITS has built momentum around its partnering activity similar to Adimab. Its first partner, Amgen, has maintained their relationship with ITS with non-exclusive access to HuTARG for fully human antibodies and other non-Ig scaffolds. With the expansion of licensing agreements to use HuTARG to discover fully human antibody therapeutics with Sanofi (5/2018), Johnson & Johnson/Janssen (8/2016), Zymeworks (8/2016), and in particular Merck (6/2016) for a deal valued at $150M.
Like most drug discovery platform companies, ITS can continue to license out molecules or potentially transition toward internal development. Given the long track record of ITS, they will probably stick with their model. When compared to Adimab and even AbCellera now, ITS’ business model still has a lot of room to improve. The new focus on differentiating on discovering antibodies against GPCRs (both agonists and antagonists - with an Ab for GPCRs not yet approved), technically challenging targets, relying on HuTARG’s ability to use single-cell functional assays to screen billions of anti-GPCR antibodies with different CDRs. Over the next few years this strategy’s effectiveness will become more apparent.
ITS has an incredible technical moat that hasn’t completely translated into a business model moat. As a result, having a deep understanding of what is important for the value of the business beyond technology at the beginning can have an enormous impact on a company’s long-term trajectory. Life sciences companies initially tend to focus on solving technical problems as they are pressing; however, the ability to design a unique business model at the formation or early phase of a business can be the difference between partnering with a handful of companies (i.e. ITS) to dozens (i.e. Adimab).