
Chimeric molecules
In line with PROTACs and the work the Crews Lab pioneered (forming Arvinas based on this work) to design bifunctional molecules to selectively degrade target proteins, work from Genentech used the same premise to enable selective de-phosphorylation. This is an extension of ~2 decades of work around chimeric molecules and offers a useful toolkit and methodology to extend the power of bifunctionality beyond degradation and phosphorylation to selective chromatin activation or glycosylation.
This analysis is meant to be the starting basis for a journal club. Traditionally, one goes through the figures and as long as the paper is not from Cell, it doesn’t take too long. The most important part is to identify the set of key experiments, either conducted or need to be done, that prove the discovery or invention. For translation, setting the corresponding milestones for the work is the next step from a technical analysis to a business one.
Key experiments:
Identify valuable targets to do experiments on - for the paper, the group focused on two oncogenic kinases, AKT and EGFR that are important drivers for numerous cancers and have phosphorylation as key factors influencing their activity.
The first step is to show the concept of brining a phosphatase close to a target protein works- the group focused on one target, AKT.
After proving this, designing better molecules is the next step. However, this is very hard from a chemistry perspective so you would want to make sure your concept works before spending the time to synthesize the chemical matter.
Expand to a new target(s) to show broader use - for the paper, the next target was EGFR.
What milestones to design a business or commercial project around this invention:
The current paper validates the idea of bringing bifunctionality to control other post-translational modifications beyond ubiquitin. To design a business, the key question is to figure out where the technical moat is. Is it the molecule design, phosphatase mapping, or something else?
What set of targets would one pursue? This is where strategic thinking and some modeling helps.
Once the targets are zeroed in on then generating in vivo is very important to further validate the technology and be in a better position for a few business tasks (i.e. partnerships, fundraising).
The premise of the paper was to bring the ability to use small molecules to selectively ubiquinate proteins to lead to degradation (PROTACs) to selectively de-phosphorylate proteins to augment their activity. Many proteins’ especially kinases’ activity are regulated by whether they are phosphorylated or not. As a result, the ability to selectively remove phosphates from proteins can inactivate targets and often whole pathways that rely on phosphorylation as the activating signal. Just as PROTACs recruit a target protein to an E3 ligase, the paper sought to design molecules to bring a target protein to a serine/threonine phosphatase:

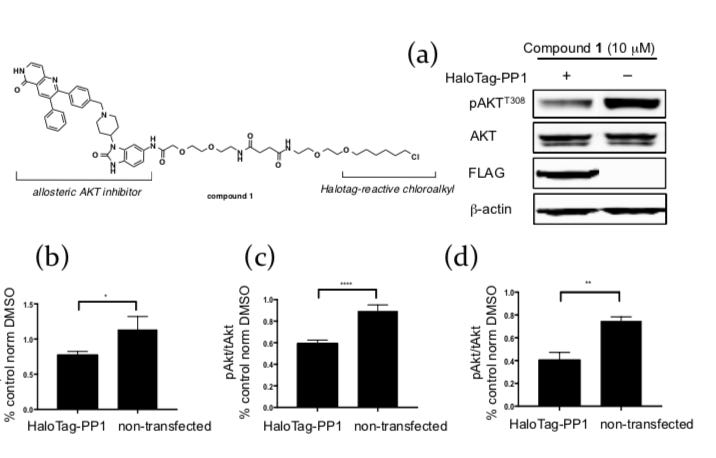
The first experiment was just to prove that recruiting a phosphatase to a protein can induce dephosphorylation. Instead of banging their heads on total synthesis of the right bifunctional molecule, the team used an off-the-shelf inhibitor of AKT, a oncogenic protein activated by phosphorylation at two residues - Thr308 and Ser473, connected to a HaloTag (a generic linker). In LNCaP cells (common adherent oncology cell line), the group transfected HaloTagged phosphatase (PP1) and treated the cells with the molecule shown above. The important readout for this step is Figure 2a - Thr308 phosphorylation levels in the cells were markedly decreased when treated with the compound compared to the control.

After showing their concept worked, the group moved toward designing a truly bifunctional molecule that can bind a target and a phosphatase without help from a tag. Thankfully another paper invented a peptide, PDP1, that selectively binds PP1. So the paper’s authors did the necessary chemistry to combine PDP1 with the AKT1 inhibitor. They ended up publishing 6 different variants (i.e. changing specific moieties) of the bifunctional molecule all with different properties. The one that ended up working really well was compound 4a (choosing Ph and Me as the R groups). That’s chemistry - you synthesis a bunch of matter and see what works. The synthesis is the hard part and can often consume 10 years of someone’s life. You think wet lab work in biology is hard; total synthesis is much harder.
The key readout is in Figure 4a where compound 4a reduces phosphorylated AKT at both sites when compared to controls. The next important important experiment was in Figure 4e where the group knocked down PPI with an siRNA rescuing phosphorylated AKT to show that the bifunctional molecule is necessary and sufficient to augment AKT phosphorylation.

After showing a proof-of-concept in AKT, the group shifted its focus on EGFR to show the broader applicability of the idea. EGFR is a major driver in several cancers and phosphorylation drives its activity. The group combined a known EGFR inhibitor (Osimertinib) with a HaloTag. Similar to the experiment done in Figure 2, the group transfected a PPI with a HaloTag and treated the cells with the molecule at the same concentration (10 μM) and time (8 hours). Expectedly, in Figure 6a, the group found that the molecule decreased levels of phosphorylated EGFR, maybe not at similar levels as the work on AKT.
This paper invents a new class of chemical matter - PhoRCs -Phosphatase Recruiting Chimeras. As a starting point, future work is around design medicinally relevant molecules and expanding the target classes. As PROTACs have a forward mechanism to tag (Ub) a target protein and degrade it, PhoRCs are reverse by nature where a target’s tag (P) is removed to limit the target’s activity. Bringing a new paradigm to medicine is really important and probably will form the basis for new projects and companies. This paper is very powerful - maybe it didn’t get media attention or trend on Twitter, but often the world’s great inventions are ignored and misunderstood. The greatest companies are often the same way initially.


