Antibody agonists trigger immune receptor signaling through local exclusion of receptor-type protein tyrosine phosphatases
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
This study investigates how antibodies can trigger signaling by immune receptors, focusing on the costimulatory receptor CD28 and the inhibitory receptor PD-1. The researchers propose and provide evidence for a mechanism whereby antibody binding leads to local exclusion of large receptor-type protein tyrosine phosphatases (RPTPs) like CD45, allowing net phosphorylation and activation of the receptors.
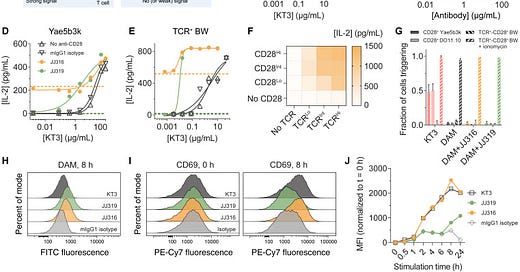
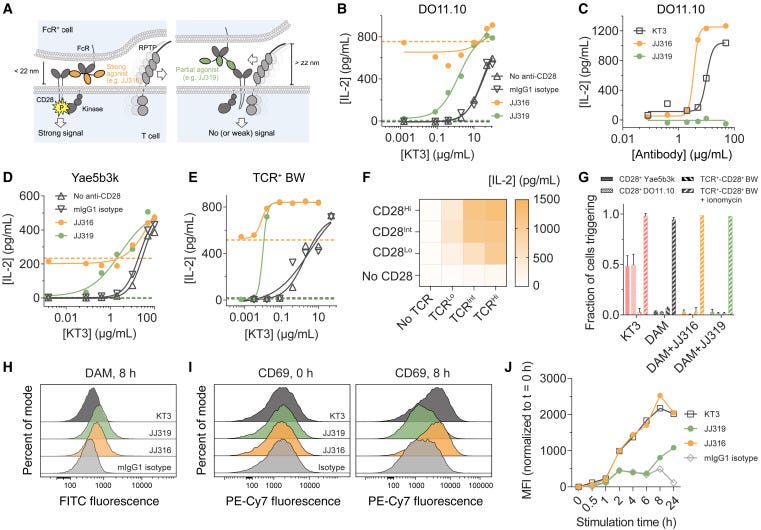
Antibody-induced signaling requires immobilization and depends on steric effects. The agonistic anti-CD28 antibody JJ316 only triggered signaling when immobilized on a surface, not when in solution. This indicates receptor cross-linking alone is insufficient. Signaling was sensitive to the dimensions of the receptor, antibody, and RPTPs. Extending the length of CD28 or the antibody reduced agonism. Truncating the large RPTPs CD45 and CD148 enhanced signaling. These results suggest antibody binding creates steric constraints that exclude large RPTPs from accessing the receptor.
Strong agonists are better at excluding RPTPs. Using total internal reflection fluorescence (TIRF) microscopy, the researchers showed the strong agonist JJ316 excluded CD45 from regions of antibody-receptor binding more effectively than the weaker agonist JJ319. This exclusion effect was more pronounced on fluid lipid bilayers compared to glass surfaces, likely due to greater protein mobility. Even modest differences in CD45 exclusion (1.1-1.9 fold on glass, ~6 fold on bilayers) correlated with large differences in signaling, suggesting T cells are sensitive to small changes in receptor phosphorylation.
Anti-PD-1 antibodies can act as agonists through the same mechanism. An anti-PD-1 antibody (clone 19) that bound near the membrane excluded CD45 and recruited the phosphatase SHP2 to PD-1, indicating receptor phosphorylation and signaling. The degree of SHP2 recruitment correlated with the antibody's ability to exclude CD45, which depended on its epitope location. Surprisingly, the PD-1 blocking antibodies nivolumab and pembrolizumab also showed some agonistic activity in certain assays, which was reduced by extending the antibody hinge region or preventing Fc receptor binding.
In mice with humanized PD-1, clone 19 and a mouse IgG1 version of nivolumab suppressed antigen-specific T cell expansion. Extending nivolumab's hinge or preventing Fc receptor binding reversed this effect, enhancing T cell expansion instead. Clone 19 suppressed delayed-type hypersensitivity and reduced disease in a systemic lupus erythematosus model, demonstrating potential therapeutic applications.
Crystal structure analysis of PD-1 bound to clone 19 showed no major structural rearrangements of the receptor, ruling out conformational change as the trigger for signaling.
This work provides a mechanistic understanding for rationally developing agonistic or blocking antibodies targeting immune receptors. For agonists, signaling strength could be tuned by selecting epitopes that alter the "gap" between cells, allowing safer targeting of activating receptors. For blocking antibodies, the goal should be to avoid RPTP exclusion or potentially to wholly exclude receptors from cell-cell contacts. The finding that current PD-1 blocking antibodies have some agonistic activity suggests their efficacy might be improved by reducing this effect. Antibody engineering to extend the hinge region or prevent Fc receptor binding could enhance PD-1 blockade.
Non-ligand blocking agonistic antibodies like clone 19 could be effective for treating autoimmune diseases by enhancing inhibitory signaling. The quantitative nature of antibody-induced signaling suggests it may be possible to titrate agonistic effects for optimal therapeutic benefit. The results support the kinetic-segregation model of immune receptor triggering, where exclusion of large phosphatases from regions of close cell-cell contact allows net phosphorylation of receptors. This mechanism may apply broadly to RPTP-sensitive immune receptors, of which there are many potential therapeutic targets.
RPTP reorganization was only studied on model surfaces, not in the context of true cell-cell contacts. While their mouse IgG1 version of nivolumab showed agonistic effects in vivo, and nivolumab/pembrolizumab biosimilars were agonistic in some in vitro assays, the extent to which the clinical antibodies have agonistic activity in humans remains uncertain. In conclusion, this study provides a mechanistic explanation for how antibodies can trigger signaling by immune receptors through local exclusion of phosphatases. This understanding has important implications for the design and optimization of therapeutic antibodies targeting these receptors, potentially leading to improved treatments for cancer and autoimmune diseases. The work also strengthens support for the kinetic-segregation model of immune receptor triggering and provides insights into the sensitive and quantitative nature of T cell signaling.
https://www.cell.com/immunity/fulltext/S1074-7613(24)00033-5