A fluorescent splice-switching mouse model enables high-throughput, sensitive quantification of antisense oligonucleotide delivery and activity
Inventors & their inventions
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company. We are excited to be in business with you — email us at info@axialvc.com
The paper presents the development and characterization of a knock-in mouse model that enables high-throughput and sensitive quantification of antisense oligonucleotide (ASO) delivery and activity. ASOs are therapeutic agents that modulate gene expression by targeting specific mRNA sequences, and they have shown promise in treating various diseases. However, assessing ASO delivery and activity in vivo has been a significant challenge, hindering the development of ASO therapeutics.
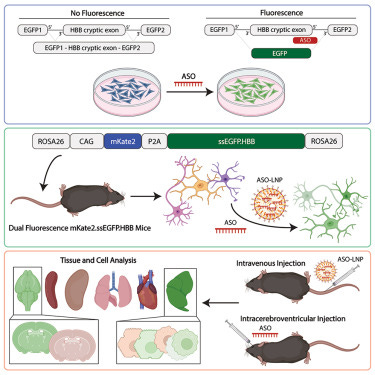
The researchers addressed this challenge by engineering a dual-fluorescence reporter system called mKate2.ssEGFP.HBB, which they integrated into the ROSA26 safe-harbor locus of mice using CRISPR-Cas9. The reporter system consists of two fluorescent proteins: mKate2, which is constitutively expressed and serves as an internal control, and EGFP (enhanced green fluorescent protein), which is alternatively spliced and expressed only in the presence of a specific ASO.
Optimization of the EGFP splice-switching reporter: first, the authors optimized the EGFP splice-switching cassette by introducing mutations that reduced background EGFP expression, ensuring minimal leakiness in the absence of ASO treatment. This improvement allowed for more accurate quantification of ASO activity.
Using primary cultures of neurons, microglia, and astrocytes derived from the knock-in mice, the researchers demonstrated that EGFP splice-switching was dependent on ASO concentration and incubation time. This in vitro system allows for high-throughput screening of ASO activity across different cell types. After intracerebroventricular (i.c.v.) injection of ASOs in the knock-in mice, the researchers observed robust EGFP splice-switching in various brain regions and cell types, including neurons, microglia, astrocytes, and oligodendrocytes. The EGFP signal was both time- and concentration-dependent, providing a quantitative readout of ASO activity and kinetics.
The researchers then used the knock-in model to assess the efficacy of different LNP formulations for ASO delivery. They demonstrated dose-dependent EGFP splice-switching in HEK293 cells treated with ASO-LNPs and observed robust EGFP induction in the liver following intravenous administration of ASO-LNPs in mice. Additionally, they isolated EGFP-positive liver cells using fluorescence-activated cell sorting (FACS), highlighting the potential for single-cell analysis.
The development of this knock-in mouse model addresses a significant limitation in ASO therapeutic development by providing a high-throughput and quantitative platform for assessing ASO delivery and activity in vivo. The model allows for the evaluation of ASO efficacy, kinetics, and cell/tissue specificity, which can facilitate the optimization of ASO design and delivery strategies. Furthermore, the ability to isolate EGFP-positive cells using FACS opens up opportunities for downstream analyses, such as single-cell transcriptomics, to investigate the molecular and cellular effects of ASO treatment at a higher resolution.
While the study primarily focused on the central nervous system (CNS) and liver, the authors acknowledge that the model can be applied to other tissues and disease indications, as the mKate2.ssEGFP.HBB transgene is widely expressed throughout the animal. One limitation of the study is that in vivo live animal imaging was limited by EGFP autofluorescence, which could potentially be addressed by using alternative fluorescent probes or luminescence readouts for ASO activity.
Overall, this study represents a significant advancement in the field of ASO therapeutics by providing a powerful tool for quantitative assessment of ASO delivery and activity in vitro and in vivo. The knock-in mouse model has the potential to accelerate the development of ASO therapeutics for various diseases by facilitating the optimization of ASO design, delivery strategies, and target specificity.
https://www.sciencedirect.com/science/article/pii/S2667237523003521?via%3Dihub